Abstract
Introduction
Impaired motor function is a major cause of disability in multiple sclerosis (MS), involving various neuroplasticity processes typically assessed by neuroimaging. This study aimed to determine whether navigated transcranial magnetic stimulation (nTMS) could also provide biomarkers of motor cortex plasticity in patients with MS (pwMS).
Methods
nTMS motor mapping was performed for hand and leg muscles bilaterally. nTMS variables included the amplitude and latency of motor evoked potentials (MEPs), corticospinal excitability measures, and the size of cortical motor maps (CMMs). Clinical assessment included disability (Expanded Disability Status Scale, EDSS), strength (MRC scale, pinch and grip), and dexterity (9-hole Pegboard Test).
Results
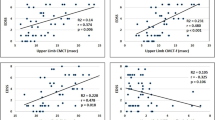
nTMS motor mapping was performed in 68 pwMS. PwMS with high disability (EDSS ≥ 3) had enlarged CMMs with less dense distribution of MEPs and various MEP parameter changes compared to pwMS with low disability (EDSS < 3). Patients with progressive MS had also various MEP parameter changes compared to pwMS with relapsing remitting form. MRC score correlated positively with MEP amplitude and negatively with MEP latency, pinch strength correlated negatively with CMM volume and dexterity with MEP latency.
Conclusions
This is the first study to perform 4-limb cortical motor mapping in pwMS using a dedicated nTMS procedure. By quantifying the cortical surface representation of a given muscle and the variability of MEP within this representation, nTMS can provide new biomarkers of motor function impairment in pwMS. Our study opens perspectives for the use of nTMS as an objective method for assessing pwMS disability in clinical practice.



Similar content being viewed by others
Data availability
Data supporting the findings are available upon reasonable request.
References
Compston A, Coles A (2008) Multiple sclerosis. Lancet 372:1502–1517. https://doi.org/10.1016/S0140-6736(08)61620-7
da Chagas LS, Sandre PC, Ribeiro e Ribeiro NCA, Marcondes H, Oliveira Silva P, Savino W (2020) Environmental signals on microglial function during brain development neuroplasticity, and diseASE. Int J Mol Sci 21:2111. https://doi.org/10.3390/ijms21062111
Li Q, Barres BA (2018) Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol 18:225–242. https://doi.org/10.1038/nri.2017.125
Rocca MA, Colombo B, Falini A, Ghezzi A, Martinelli V, Scotti G et al (2005) Cortical adaptation in patients with MS: a cross-sectional functional MRI study of disease phenotypes. Lancet Neurol 4:618–626. https://doi.org/10.1016/S1474-4422(05)70171-X
Lefaucheur J-P (2010) Why image-guided navigation becomes essential in the practice of transcranial magnetic stimulation. Neurophysiol Clin 40:1–5. https://doi.org/10.1016/j.neucli.2009.10.004
Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G et al (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 17:162–173. https://doi.org/10.1016/S1474-4422(17)30470-2
Krieg SM, Lioumis P, Mäkelä JP, Wilenius J, Karhu J, Hannula H et al (2017) Protocol for motor and language mapping by navigated TMS in patients and healthy volunteers; workshop report. Acta Neurochir (Wien) 159:1187–1195. https://doi.org/10.1007/s00701-017-3187-z
Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin J-C, Pujol S et al (2012) 3D Slicer as an image computing platform for the quantitative imaging network. Magn Reson Imaging 30:1323–1341. https://doi.org/10.1016/j.mri.2012.05.001
Pieper S, Halle M, Kikinis R (2004) 3D Slicer. In 2004 2nd IEEE International Symposium on Biomedical Imaging: Macro to Nano (IEEE Cat No 04EX821) 2:632–635. https://doi.org/10.1109/ISBI.2004.1398617
Egger C, Opfer R, Wang C, Kepp T, Sormani MP, Spies L et al (2016) MRI FLAIR lesion segmentation in multiple sclerosis: Does automated segmentation hold up with manual annotation? Neuroimage Clin 13:264–270. https://doi.org/10.1016/j.nicl.2016.11.020
Friston KJ, Ashburner J, Kiebel SJ, Nichols TE, Penny WD (2007) Statistical Parametric Mapping: The Analysis of Functional Brain Images. Academic Press. https://doi.org/10.1016/B978-0-12-372560-8.X5000-1
Fan L, Li H, Zhuo J, Zhang Y, Wang J, Chen L et al (2016) The human brainnetome atlas: a new brain atlas based on connectional architecture. Cereb Cortex 26:3508–3526. https://doi.org/10.1093/cercor/bhw157
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33:1444–1452
Meca-Lallana V, Brañas-Pampillón M, Higueras Y, Candeliere-Merlicco A, Aladro-Benito Y, Rodríguez-De la Fuente O et al (2019) Assessing fatigue in multiple sclerosis: psychometric properties of the five-item Modified Fatigue Impact Scale (MFIS-5). Mult Scler J Exp Transl Clin 5:2055217319887987. https://doi.org/10.1177/2055217319887987
Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand 67:361–370
Smith A (1982) Symbol digit modalities test (SDMT). Manual (Revised). Western Psychological Services, Los Angeles
Llinàs-Reglà J, Vilalta-Franch J, López-Pousa S, Calvó-Perxas L, Torrents Rodas D, Garre-Olmo J (2017) The trail making test. Assessment 24:183–196. https://doi.org/10.1177/1073191115602552
Compston A (2010) Aids to the investigation of peripheral nerve injuries. Medical Research Council: Nerve Injuries Research Committee. His Majesty’s Stationery Office: 1942; pp. 48 (iii) and 74 figures and 7 diagrams; with aids to the examination of the peripheral nervous system. By Michael O’Brien for the Guarantors of Brain. Saunders Elsevier: 2010; pp. [8] 64 and 94 Figures. Brain 133:2838–2844. https://doi.org/10.1093/brain/awq270
Oldfield RC (1971) The assessment and analysis of handedness: the edinburgh inventory. Neuropsychologia 9:97–113
Elias LJ, Bryden MP, Bulman-Fleming MB (1998) Footedness is a better predictor than is handedness of emotional lateralization. Neuropsychologia 36:37–43. https://doi.org/10.1016/s0028-3932(97)00107-3
Mathiowetz V, Kashman N, Volland G, Weber K, Dowe M, Rogers S (1985) Grip and pinch strength: normative data for adults. Arch Phys Med Rehabil 66:69–74
Truong CTL, Le HV, Kamauu AW, Holmen JR, Fillmore CL, Kobayashi MG et al (2021) Creating a real-world data, united states healthcare claims-based adaptation of Kurtzke functional systems scores for assessing multiple sclerosis severity and progression. Adv Ther 38:4786–4797. https://doi.org/10.1007/s12325-021-01858-9
Sayao A-L, Devonshire V, Tremlett H (2007) Longitudinal follow-up of “benign” multiple sclerosis at 20 years. Neurology 68:496–500. https://doi.org/10.1212/01.wnl.0000253185.03943.66
Thickbroom GW, Byrnes ML, Archer SA, Kermode AG, Mastaglia FL (2005) Corticomotor organisation and motor function in multiple sclerosis. J Neurol 252:765–771. https://doi.org/10.1007/s00415-005-0728-9
Chieffo R, Straffi L, Inuggi A, Coppi E, Moiola L, Martinelli V et al (2019) Changes in cortical motor outputs after a motor relapse of multiple sclerosis. Mult Scler J Exp Transl Clin 5:2055217319866480. https://doi.org/10.1177/2055217319866480
Weiss Lucas C, Tursunova I, Neuschmelting V, Nettekoven C, Oros-Peusquens A-M, Stoffels G et al (2017) Functional MRI vs. navigated TMS to optimize M1 seed volume delineation for DTI tractography. A prospective study in patients with brain tumours adjacent to the corticospinal tract. Neuroimage Clin 13:297–309. https://doi.org/10.1016/j.nicl.2016.11.022
Lefaucheur J-P, Picht T (2016) The value of preoperative functional cortical mapping using navigated TMS. Neurophysiol Clin 46:125–133. https://doi.org/10.1016/j.neucli.2016.05.001
Kraus D, Gharabaghi A (2015) Projecting navigated TMS sites on the Gyral anatomy decreases inter-subject variability of cortical motor maps. Brain Stimul 8:831–837. https://doi.org/10.1016/j.brs.2015.03.006
Julkunen P (2014) Methods for estimating cortical motor representation size and location in navigated transcranial magnetic stimulation. J Neurosci Methods 232:125–133. https://doi.org/10.1016/j.jneumeth.2014.05.020
Rocca MA, Filippi M (2017) Functional reorganization is a maladaptive response to injury–YES. Mult Scler 23:191–193. https://doi.org/10.1177/1352458516667242
Filippi M, Agosta F (2009) Magnetic resonance techniques to quantify tissue damage, tissue repair, and functional cortical reorganization in multiple sclerosis. Prog Brain Res 175:465–482. https://doi.org/10.1016/S0079-6123(09)17531-3
Laura DG, Silvia T, Nikolaos P, Patrizia P (2018) The role of fMRI in the Assessment Of Neuroplasticity in MS: a systematic review. Neural Plast 2018:3419871. https://doi.org/10.1155/2018/3419871
Filippi M, Preziosa P, Rocca MA (2019) Brain mapping in multiple sclerosis: lessons learned about the human brain. Neuroimage 190:32–45. https://doi.org/10.1016/j.neuroimage.2017.09.021
Tavazzi E, Cazzoli M, Pirastru A, Blasi V, Rovaris M, Bergsland N et al (2021) Neuroplasticity and motor rehabilitation in multiple sclerosis: a systematic review on MRI markers of functional and structural changes. Front Neurosci 15:707675. https://doi.org/10.3389/fnins.2021.707675
Mezzapesa DM, Rocca MA, Rodegher M, Comi G, Filippi M (2008) Functional cortical changes of the sensorimotor network are associated with clinical recovery in multiple sclerosis. Hum Brain Mapp 29:562–573. https://doi.org/10.1002/hbm.20418
Enzinger C, Pinter D, Rocca MA, De Luca J, Sastre-Garriga J, Audoin B et al (2016) Longitudinal fMRI studies: exploring brain plasticity and repair in MS. Mult Scler 22:269–278. https://doi.org/10.1177/1352458515619781
Stampanoni Bassi M, Mori F, Buttari F, Marfia GA, Sancesario A, Centonze D et al (2017) Neurophysiology of synaptic functioning in multiple sclerosis. Clin Neurophysiol 128:1148–1157. https://doi.org/10.1016/j.clinph.2017.04.006
Lenzi D, Conte A, Mainero C, Frasca V, Fubelli F, Totaro P et al (2007) Effect of corpus callosum damage on ipsilateral motor activation in patients with multiple sclerosis: a functional and anatomical study. Hum Brain Mapp 28:636–644. https://doi.org/10.1002/hbm.20305
Kiers L, Cros D, Chiappa KH, Fang J (1993) Variability of motor potentials evoked by transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol 89:415–423. https://doi.org/10.1016/0168-5597(93)90115-6
Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R et al (2015) Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee Clin Neurophysiol 126:1071–1107. https://doi.org/10.1016/j.clinph.2015.02.001
Reijonen J, Säisänen L, Könönen M, Mohammadi A, Julkunen P (2020) The effect of coil placement and orientation on the assessment of focal excitability in motor mapping with navigated transcranial magnetic stimulation. J Neurosci Methods 331:108521. https://doi.org/10.1016/j.jneumeth.2019.108521
Opitz A, Legon W, Rowlands A, Bickel WK, Paulus W, Tyler WJ (2013) Physiological observations validate finite element models for estimating subject-specific electric field distributions induced by transcranial magnetic stimulation of the human motor cortex. Neuroimage 81:253–264. https://doi.org/10.1016/j.neuroimage.2013.04.067
Bergmann TO, Mölle M, Schmidt MA, Lindner C, Marshall L, Born J et al (2012) EEG-guided transcranial magnetic stimulation reveals rapid shifts in motor cortical excitability during the human sleep slow oscillation. J Neurosci 32:243–253. https://doi.org/10.1523/JNEUROSCI.4792-11.2012
Keil J, Timm J, Sanmiguel I, Schulz H, Obleser J, Schönwiesner M (2014) Cortical brain states and corticospinal synchronization influence TMS-evoked motor potentials. J Neurophysiol 111:513–519. https://doi.org/10.1152/jn.00387.2013
de Goede AA, van Putten MJAM (2019) Infraslow activity as a potential modulator of corticomotor excitability. J Neurophysiol 122:325–335. https://doi.org/10.1152/jn.00663.2018
Potter-Baker KA, Lin Y-L, Machado AG, Conforto AB, Cunningham DA, Sankarasubramanian V et al (2018) Variability of motor evoked potentials in stroke explained by corticospinal pathway integrity. Brain Stimul 11:929–931. https://doi.org/10.1016/j.brs.2018.03.004
Sollmann N, Bulubas L, Tanigawa N, Zimmer C, Meyer B, Krieg SM (2017) The variability of motor evoked potential latencies in neurosurgical motor mapping by preoperative navigated transcranial magnetic stimulation. BMC Neurosci 18:5. https://doi.org/10.1186/s12868-016-0321-4
Picht T, Strack V, Schulz J, Zdunczyk A, Frey D, Schmidt S et al (2012) Assessing the functional status of the motor system in brain tumor patients using transcranial magnetic stimulation. Acta Neurochir (Wien) 154:2075–2081. https://doi.org/10.1007/s00701-012-1494-y
Britton TC, Meyer BU, Benecke R (1991) Variability of cortically evoked motor responses in multiple sclerosis. Electroencephalogr Clin Neurophysiol 81:186–194. https://doi.org/10.1016/0168-5597(91)90071-5
Thickbroom GW, Byrnes ML, Mastaglia FL (1999) A model of the effect of MEP amplitude variation on the accuracy of TMS mapping. Clin Neurophysiol 110:941–943. https://doi.org/10.1016/s1388-2457(98)00080-7
Simpson M, Macdonell R (2015) The use of transcranial magnetic stimulation in diagnosis, prognostication and treatment evaluation in multiple sclerosis. Mult Scler Relat Disord 4:430–436. https://doi.org/10.1016/j.msard.2015.06.014
Snow NJ, Wadden KP, Chaves AR, Ploughman M (2019) Transcranial magnetic stimulation as a potential biomarker in multiple sclerosis: a systematic review with recommendations for future research. Neural Plast 2019:6430596. https://doi.org/10.1155/2019/6430596
Vucic S, Stanley Chen K-H, Kiernan MC, Hallett M, Benninger DH, Di Lazzaro V et al (2023) Clinical diagnostic utility of transcranial magnetic stimulation in neurological disorders. Updated report of an IFCN committee. Clin Neurophysiol 150:131–175. https://doi.org/10.1016/j.clinph.2023.03.010
Lefaucheur J-P, Drouot X, Ménard-Lefaucheur I, Keravel Y, Nguyen JP (2006) Motor cortex rTMS restores defective intracortical inhibition in chronic neuropathic pain. Neurology 67:1568–1574. https://doi.org/10.1212/01.wnl.0000242731.10074.3c
Krieg SM, Shiban E, Buchmann N, Gempt J, Foerschler A, Meyer B et al (2012) Utility of presurgical navigated transcranial magnetic brain stimulation for the resection of tumors in eloquent motor areas. J Neurosurg 116:994–1001. https://doi.org/10.3171/2011.12.JNS111524
Tarapore PE, Picht T, Bulubas L, Shin Y, Kulchytska N, Meyer B et al (2016) Safety and tolerability of navigated TMS for preoperative mapping in neurosurgical patients. Clin Neurophysiol 127:1895–1900. https://doi.org/10.1016/j.clinph.2015.11.042
Acknowledgements
The authors would like to thank the Observatoire Français de la Sclérose en Plaques (OFSEP, http://www.ofsep.org/fr/), which is supported by a grant provided by the French government and administered by the Agence Nationale de la Recherche, within the framework of the ‘Investments for the Future’ programme (reference ANR‐10‐COHO‐002), the Eugène Devic EDMUS Foundation against Multiple Sclerosis and the ARSEP Foundation.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
Benjamin Bardel: conceptualization of the study, main contribution to data collection and analyses, acquisition of neurophysiological data, programming, statistical analysis, drafted the manuscript; Alain Créange: main contribution to patient recruitment, data collection, interpretation of data, revision of the manuscript; Nathalie Bonardet, Mickael Zedet, Abir Wahab: contribution to patient recruitment and data collection; Blanche Bapst: contribution to data collection and analyses; Samar Ayache: main contribution to concept and design of study; Jean-Pascal Lefaucheur: supervision of the study, validation of results, supervision of writing the article, revising the manuscript critically for important intellectual content.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bardel, B., Créange, A., Bonardet, N. et al. Motor function in multiple sclerosis assessed by navigated transcranial magnetic stimulation mapping. J Neurol (2024). https://doi.org/10.1007/s00415-024-12398-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00415-024-12398-x