Abstract
Objective
Half of ALS patients are cognitively and/or behaviourally impaired. As cognition/behaviour and cerebral glucose metabolism can be correlated by means of 18F-Fluorodeoxyglucose positron emission tomography (FDG-PET), we aimed to utilise FDG-PET, first, to replicate group-level differences in glucose metabolism between non-demented ALS patients separated into non-impaired (ALSni), cognitively impaired (ALSci), behaviourally impaired (ALSbi), and cognitively and behaviourally impaired (ALScbi) groups; second, to investigate glucose metabolism and performance in various cognitive domains; and third, to examine the impact of partial volume effects correction (PVEC) of the FDG-PET data on the results.
Methods
We analysed neuropsychological, clinical, and imaging data from 67 ALS patients (30 ALSni, 21 ALSci, 5 ALSbi, and 11 ALScbi). Cognition was assessed with the Edinburgh Cognitive and Behavioural ALS Screen, and two social cognition tests. FDG-PET and structural MRI scans were acquired for each patient. Voxel-based statistical analyses were undertaken on grey matter volume (GMV) and non-corrected vs. PVE-corrected FDG-PET scans.
Results
ALSci and ALScbi had lower cognitive scores than ALSni. In contrast to both ALSni and ALSci, ALScbi showed widespread hypometabolism in the superior- and middle-frontal gyri in addition to the right temporal pole. Correlations were observed between the GMV, the FDG-PET signal, and various cognitive scores. The FDG-PET results were largely unaffected by PVEC.
Interpretation
Our study identified widespread differences in hypometabolism in the ALScbi-ni but not in the ALSci-ni group comparison, raising the possibility that cerebral metabolism may be more closely related to the presence of behavioural changes than to mild cognitive deficits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative motor neuron disorder. Besides rapidly advancing motor impairment, up to 50% of people living with ALS are cognitively impaired, up to 70% show behavioural changes, and up to 15% meet the criteria for a frontotemporal dementia [1]. The cognitive domains characteristically affected in ALS include verbal fluency, executive functioning, social cognition, and language [2, 3]. Behavioural changes include apathy, irritability, and increased self-centeredness [1, 4, 5].
Positron emission tomography (PET) imaging with the radiotracer 2-[18F]Fluorodeoxyglucose (FDG) provides an estimation of glucose uptake by neurons and astrocytes [6, 7], enabling the investigation of differences in regional metabolism. The typical pattern of glucose hypo- and hypermetabolism in ALS has been well documented, with the most consistently reported hypometabolic regions being the motor cortex and prefrontal cortex [8,9,10]. In terms of hypermetabolism, previous studies have reported alterations in the cerebellum, occipital cortex, midbrain, and medial temporal cortex [8,9,10,11,12].
Associations have been reported between cognitive status and metabolic changes in ALS patients: more extensive and severe frontal hypometabolism has been correlated with an increased severity of cognitive and behavioural impairments [13]. Besides group differences between ALS patients with or without cognitive impairments, correlations have been described between metabolism in specific brain regions and performance in cognitive tasks. An impaired cognitive theory of mind has been linked to increased metabolism in the left fusiform gyrus [10], and decreased metabolism in the prefrontal cortex [14], the supplementary motor area [14], and the left inferior frontal gyrus [15]. Weaker episodic memory performance has been associated with increased metabolism in the hippocampus and left parahippocampal gyrus [10]. Concerning behavioural impairment, the increasing severity of apathy has been negatively associated with metabolism in the prefrontal cortex, premotor cortex, anterior cingulate gyrus, and insula as well as positively associated with metabolism in the cerebellum and pons [16]. However, evidence is still scarce on associations between glucose metabolism and specific cognitive domains relevant in ALS such as executive functioning, verbal fluency, and social cognition.
An important consideration for PET analyses are partial volume effects (PVEs). The radiotracer signal measured in a given brain region reflects a combination of the true regional signal and the signal from neighbouring tissues or CSF, leading to blurring [17]. When the signal in grey matter is higher than in white matter or CSF, as is the case with FDG-PET, PVEs lead to an underestimation of the true grey matter signal. Besides scanner resolution, the extent of PVEs is further dependent on the size of the measured region and the presence of atrophy [17]. The majority of FDG-PET studies did not include a PVE correction (PVEC) [8, 9, 11, 13, 16] based on the assumption that metabolic measurements in ALS are relatively unaffected by PVEs, but this assumption has not yet widely been tested.
The aims of this study were thus (1) to replicate findings on differences in glucose metabolism and grey matter volume between ALS patients with and without cognitive or behavioural impairment, (2) to investigate associations of glucose metabolism and grey matter volume with performance in ALS-related cognitive domains, and (3) to examine the impact of PVEC of the FDG-PET data on the group differences and domain-specific associations.
Materials and methods
Participants
67 patients were recruited by the Department of Neurology of the Rostock University Medical Centre. They were diagnosed according to the modified El Escorial criteria [18] and characterised using the revised ALS Functional Rating Scale (ALSFRS-R) [19]. The exclusion criteria included a history of brain injury, epilepsy, psychiatric illness, or a diagnosis of dementia [20].
Neuropsychological assessment
The neuropsychological assessment included the German versions of the Edinburgh cognitive and behavioural ALS screen (ECAS) [21], Faux Pas test [22], the Facial Recognition Task [23], and the informant version of the Frontal System Behaviour Scale (FrSBe) [24]. ECAS assesses five cognitive domains with multiple subtests [25], the Faux-pas test is an advanced theory of mind task, and the Facial Recognition Task measures the ability to identify the six basic emotions based on facial expressions. The FrSBe assesses behavioural changes in apathy, disinhibition, and executive dysfunction.
Cognitive and behavioural impairments were classified according to the revised Strong criteria [26] as follows: patients without cognitive and/or behavioural impairment (ALSni), patients with cognitive impairment (ALSci), patients with behavioural impairment (ALSbi) and patients with cognitive and behavioural impairment (ALScbi). Cognitive impairment was defined as a deficient score in the verbal fluency, executive functions, or language domain based on age- and education-adjusted ECAS-norms from a Swiss and German sample [21]. Norms from a French sample were used for Faux Pas test and Facial Recognition Test [27]. The behavioural classification was based on either or both the age- and education-normed informant version of FrSBe [24], and the clinical observation of an experienced neuropsychologist.
Neuroimaging data acquisition
Structural MRI scans were taken on two scanners and FDG-PET scans were acquired on the same scanner at the Rostock University Medical Centre (Rostock, Germany). High-resolution T1-weighted anatomical images were obtained on a 3 T Siemens Skyra Fit scanner (2018–2022) or on a Siemens Vida scanner (from 2022 onwards) using the magnetization-prepared rapid gradient echo (MPRAGE) sequence with the following parameters: 192 sagittal slices, field of view = 256 × 256 mm2, image matrix = 256 × 256, isotropic voxel size = 1 mm3, echo time = 4.37 ms, repetition time = 2500 ms, and flip angle = 7°.
The patients underwent dynamic PET imaging (4 × 5 min) of the brain using a Gemini TF 16 scanner (Philips Healthcare) at 30 min after the injection of 199 ± 18 MBq FDG. Prior to PET imaging, an auxiliary CT scan (120 kVp, 30 mAs) was performed. To minimise the influence of motion artefacts, the four dynamic PET frames were examined for possible head movements of the patient. Conspicuous frames were excluded, and finally, a static PET data set, representing at least 10 min acquisition time, was reconstructed using the manufacturer's proprietary BLOB-OS reconstruction algorithm (3 iterations, 31 subsets) corrected for randoms, scatter, decay, and attenuation using information from the auxiliary CT.
Neuroimaging preprocessing
All imaging data were preprocessed in SPM12 (https://www.fil.ion.ucl.ac.uk/spm/) using Matlab.
MRI: The MRI scans were skull-stripped, segmented, and spatially normalised to the MNI152 non-linear asymmetric 2009 template using Dartel registration in the CAT12 toolbox [28]. The grey matter (GM), white matter (WM), and CSF segments in addition to the forward and inverse deformation fields were saved. The scans were smoothed with a 8 mm kernel and submitted to statistical analysis. In addition, an explicit GM mask was created by keeping GM voxels that overlap in at least 50% of the spatially normalised scans and applied in all statistical analyses.
FDG-PET: The FDG-PET scan was coregistered to the participant’s MRI scan and preprocessed both with and without PVEC according to the Müller-Gärtner [29] method using custom Matlab scripts to enable a comparison of results. GM, WM, and CSF segments from the MRI preprocessing were smoothed with a 4.8 mm kernel, accounting for the spatial resolution of the used PET-system [30], and used in the PVEC procedure. A GM threshold of 0.5 was incorporated, and trilinear interpolation was used. For the PVEC procedure, WM and CSF signal was estimated for each individual patient by thresholding their WM and CSF segments at 0.99 and averaging the signal in the resulting mask. The output of PVEC was a map of corrected FDG signal restricted to GM. Negative voxels were masked-out, and to compare results with and without PVEC the negative and non-GM voxels were also masked-out in the non-corrected FDG-PET (NC-FDG) scans. Intensity normalization on both PVEC-FDG and NC-FDG scans was carried out by dividing the signal of each voxel by the average FDG signal within GM [13, 15, 16]. This intensity normalisation approach impacts the interpretation of hypermetabolism: when global metabolism is reduced throughout widespread hypometabolic regions, then clusters of hypermetabolism may instead indicate a relative preservation of metabolism. Subsequently, all scans were spatially normalised to MNI space by applying forward deformation fields from MRI preprocessing step with trilinear interpolation. All scans were smoothed with a 10 mm kernel and submitted to statistical analysis.
Statistical analysis
The whole sample was characterised by various demographic and clinical data and equivalence of the groups was compared using Chi-squared tests for categorical variables and Analysis of Variance (ANOVA) for continuous variables in the Statistical Product and Service Solutions (SPSS, version 28.0). Kruskal–Wallis tests were used to compare the groups on individual cognitive domains. For cognitive domains where the groups differed, individual subtest scores contributing to the domain score were also compared. Mann–Whitney U tests were used for all post hoc comparisons.
Group differences in PVEC-FDG data, NC-FDG data, and GM volume were tested in a voxel-based manner with one-way ANOVA in SPM12 using Matlab. Age and sex were included as covariates in all analyses, and the total intracranial volume (TIV) was included in GM volume analysis. All possible t-contrasts were tested. Further voxel-based regression analyses on the PVEC-FDG signal, NC-FDG signal, and GM volume were specified in SPM12 for single cognitive domains and subtest scores that differed in the preceding neuropsychology analyses. Age, sex, and years of formal education were included as covariates, and the total intracranial volume (TIV) was included in GM volume analysis.
In addition, corresponding analyses were carried out using partial least squares (PLS) regression with the PLS application in Matlab [31]. This method makes use of covariance between voxels and yields latent variables (LVs) that each identify a pattern of brain regions that covary with some external measure [32]. The significance of each LV was determined with 100 permutation tests at a threshold of p < 0.05. Each voxel has a salience on each LV. To determine the reliability of these voxel saliences, we used the bootstrap estimation with 100 iterations and a threshold of p < 0.05 for the resulting bootstrap ratios. We examined all group contrasts in addition to the associations with performance on cognitive domains that differed in the preceding neuropsychology analyses using the PVEC-FDG and NC-FDG data.
Results
Demographic and clinical characteristics
The clinical and demographic information for our sample can be found in Table 1. Based on the small group size of ALSbi (n = 5), they were excluded from the group analysis. The equivalence of the groups on different variables was tested using Chi-squared tests for categorical and ANOVAs for quantitative variables.
Neuropsychological data
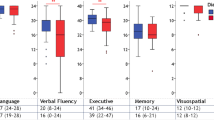
The neuropsychological data are summarised in Table 2. The three groups were compared using Kruskal–Wallis and Mann–Whitney tests. For single cognitive domains where the groups differed, subtest scores were also compared. The ALSni and ALSci patients differed on the cognitive domains of language, executive functions, verbal fluency, and memory, whereas differences between ALSni and ALScbi patients were observable on executive functions, verbal fluency, and memory. No differences in cognitive scores were observed between ALSci and ALScbi patients.
Group analyses
Groups were compared on GM volume, PVEC-FDG data, and NC-FDG data in a voxel-based manner. The height threshold was set at p < 0.001, p < 0.05 FWE-corrected at cluster level. If no clusters were detected, an exploratory analysis at a lenient threshold of p < 0.001 and a minimum cluster size of 100 voxels was undertaken (supplementary Fig. 1). The FWE-corrected results are reported below and both FWE-corrected and exploratory findings can be found in Tables 3 and 4. In addition, PLS analyses of the contrasts were carried out.
ALSni vs ALSci
No clusters were detected in GM volume, PVEC-FDG, nor the NC-FDG analysis. No differences could be detected in PLS analyses with both PVEC-FDG data (LV = 93.88, p = 0.574) and NC-FDG data (LV = 92.18, p = 0.614).
ALSni vs ALScbi
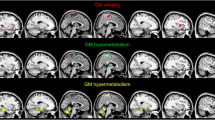
For PVEC-FDG and NC-FDG data, ALScbi patients showed hypometabolic clusters in the left superior frontal gyrus and right temporal pole. In PVEC-FDG analyses, the ALScbi group further showed hypometabolism in the right middle-frontal gyrus, and hypermetabolism in right superior occipital gyrus. The clusters are listed in Table 3 and portrayed on Fig. 1. The PLS analyses corroborated these results by identifying a similar metabolic pattern in both PVEC-FDG data (LV = 135.69, p < 0.001) and NC-FDG data (LV = 135.72, p < 0.001).
Clusters of relative hypo- and hypermetabolism per group comparison. The FDG-PET signal is relative to the average FDG-tracer uptake in GM. Height threshold is p < 0.001 and all data are p < 0.05 FWE-corrected at cluster level. ALSni not impaired; ALSci, cognitively impaired; ALScbi cognitively and behaviourally impaired; PVEC-FDG PVE-corrected FDG-PET data; NC-FDG non-corrected FDG-PET data
ALSci vs ALScbi
In PVEC-FDG and NC-FDG analyses, the ALScbi group showed a cluster of relative hypermetabolism in the left cuneus. The clusters are listed in Table 4 and portrayed on Fig. 1. In the PLS analyses, the difference was on the threshold of significance based on both the NC-FDG data (LV = 104.61, p = 0.050) and the PVEC-FDG data (LV = 99.71, p = 0.059).
Associations with single cognitive domains and subtests
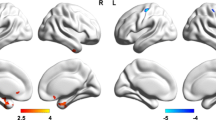
In the PVEC-FDG and NC-FDG analyses, no clusters were detected with FWE-correction at cluster level. Further exploratory analyses at a height threshold of p < 0.001 and a minimum cluster size of 100 voxels identified the following associations between glucose metabolism and single cognitive domains and their subtests (Fig. 2). All correlations were positive unless otherwise specified. The domain score of executive functioning correlated with metabolism in the left middle-frontal gyrus (NC-FDG), whereas its subtest inhibition correlated with left lingual gyrus metabolism (NC-FDG), and the subtest alternation with metabolism in the left lobule VIII of the cerebellum (NC-FDG), the left precentral gyrus (PVEC-FDG), and the left middle temporal gyrus (PVEC-FDG). The verbal fluency subtest of S-words correlated with metabolism in the bilateral superior frontal (PVEC-FDG and NC-FDG), left precentral (PVEC-FDG), and right inferior orbital gyrus (NC-FDG). Furthermore, negative associations were observed between the S-words score and metabolism in the bilateral crus I of the cerebellum. Memory performance was associated with metabolism in the right precuneus (PVEC-FDG), and the left fusiform gyrus (PVEC-FDG and NC-FDG) and the subtest of immediate recall was associated with the right precuneus (PVEC-FDG and NC-FDG) and the left middle temporal gyrus metabolism (PVEC-FDG and NC-FDG). Faux Pas test scores correlated with metabolism in the right middle-frontal gyrus (PVEC-FDG and NC-FDG) and right precentral gyrus (PVEC-FDG). None of the PLS analyses with PVEC-FDG or NC-FDG data neared significance (data not shown). Clusters of GM volume are outlined in supplementary Table 1.
Discussion
The aim of this study was to investigate the associations between cerebral glucose metabolism and cognition in ALS using group comparisons and regression analyses. In accordance with previous work in the field [13], we found that concurrent cognitive and behavioural impairment were associated with widespread changes in glucose metabolism. Relative to the average cortical FDG-PET signal, hypometabolism in the superior frontal gyri, middle-frontal gyri, and temporal pole in addition to relative hypermetabolism in the superior occipital gyrus was detected in ALScbi when compared to ALSni. In exploratory analyses without FWE-correction, the comparison of ALScbi to ALSci identified a similar pattern of metabolism changes where the location of the clusters persisted, but their extent was conspicuously limited. This pattern did not emerge for the group comparison of ALSci to ALSni, even in the exploratory analysis without FWE-correction. The result of the partial least squares (PLS) analysis further corroborated these group comparison findings. The FDG-PET signal was intensity normalised using the average FDG signal in GM, which impacts the interpretation of hypermetabolic clusters. In the case of widespread hypometabolic areas leading to a reduction in global metabolism, the co-occurring clusters of hypermetabolism should be interpreted as a relative preservation of metabolism.
In contrast to both our expectations and a previous publication [13], no clusters of hypometabolism survived the FWE-correction in ALSci compared to ALSni patients in the current sample. One possible explanation for this lack of stark differences may be the heterogeneous composition of the ALSci as defined by Strong [26], since this category includes patients with mild deficits in either verbal fluency, executive functioning, or language, and those impairments may have different neural substrates. A further consideration is the use of the ECAS [25] instead of a comprehensive neuropsychological test battery (owing to both its feasibility and its lower burden for patients). The prevalence of deficits in ALS-specific cognitive functions (fluency, executive, and language) in our sample was comparable to the original publication, which was based on and validated in UK samples [25]. However, recent research has highlighted how crucial the choice of population norms and statistical methods are in determining the prevalence of cognitive impairment based on the ECAS [33]. The results of the group comparisons in our sample are, therefore, inherently dependent on the available age- and education-adjusted German ECAS-norms [21]. This raises questions of eligibility to directly compare our results with other cohorts and has implications for the generalisation of our findings.
As would be expected, ALSni had higher test scores than both ALSci and ALScbi groups in the majority of cognitive domains, and our sample showed no significant differences in cognitive scores between ALSci and ALScbi. Behavioural changes were assessed with the FrSBe test [24], which comprises three domains: apathy, disinhibition, and executive dysfunction. As anticipated, only the ALScbi group differed from ALSni and ALSci on the total score and the apathy subscale of the test. These neuropsychological results provide context for interpreting the glucose metabolism findings. The explorative non-FWE-corrected comparison of ALSci to ALSni revealed one relatively small hypometabolic cluster in the precentral gyrus despite significant differences between the groups in the majority of assessed cognitive domains. Furthermore, the explorative non-FWE-corrected comparison of ALScbi to ALSci showed a similar distribution of hypo- and hypermetabolic clusters as the FWE-corrected ALScbi-ALSni comparison despite a lack of group differences between ALScbi and ALSci on cognitive scores. Alterations in glucose metabolism may thus be more closely related to the presence of behavioural changes than mild cognitive impairment. However, as behavioural changes cannot be completely separated from cognitive changes, particularly changes in inhibitory control and executive functioning, this somewhat limits the interpretation of our results. Further investigations are required to assess the comparative effects of glucose metabolism on cognitive and behavioural symptoms in ALS.
We observed significant differences in ALScbi when compared to ALSni regarding hypometabolism: the clusters were strongly asymmetric, and largely restricted to the frontal and temporal lobes of the right hemisphere. The notion that impaired behaviour is associated with changes in cerebral glucose metabolism has also been demonstrated by Canosa et al. [16]. Though in contrast to our markedly right hemispheric clusters, their findings showed bihemispheric clusters of negative correlation between glucose metabolism and apathy as determined by a subscale of FrSBe. Whereas their study focussed specifically on correlations of apathy, our study considered behavioural impairment in a more generalised manner. The right lateralisation of hypometabolism in behaviourally impaired ALS in this study is in accord with a tendency for right hemispheric dominance in emotional functioning in the neurobiological literature [34].
Concerning voxel-wise analyses of FDG-PET data respective to individual cognitive domains and their subtests, numerous positive correlations could be observed in the explorative SPM analyses without FWE-correction (height threshold of p < 0.001, a minimum cluster size of 100 voxels), whereas corresponding analyses utilising the PLS approach indicated a lack of associations. The diverging nature of these findings suggests an absence of strong correlations between cognitive performance and glucose metabolism at rest. Furthermore, only explorative analyses at a lenient threshold yielded associations with GM volume, which is in agreement with a previous publication [35] that reported small clusters of correlations between GM volume and ECAS scores. Therefore, possible associations between cognition and FDG-PET and GM, if present, seem to be weak.
Several studies reported applying a PVEC [10, 14, 15] and others did not [8, 9, 11, 13, 16]. Here, we directly compared the results of FDG-PET analyses with and without PVEC. The analyses with and without PVEC led to similar results. The clusters identified with both approaches overlapped to a large degree, and the LVs and their significance in the PLS analyses were similar. The influence of PVEs on FDG-PET signals seems to be negligible in ALS samples, and carrying out a PVEC does not seem to affect the results.
We need to consider several limitations of this study. First, a larger sample size would provide higher statistical power to better detect small effects and to give more confidence in interpreting negative findings as absence of true effects. Furthermore, a comprehensive neuropsychological test battery and/or population norms based on larger samples could have yielded more accurate categorisations between the cognitive groups in addition to a more detailed assessment of individual cognitive domains in contrast to the ECAS assessment [10, 13, 15, 36]. Unfortunately, the impact of motor symptoms on test performance cannot be controlled for many cognitive tests and therefore limits the validity of such test findings in ALS patients. The ECAS is able to assess cognition independent of the severity of motor symptoms and has therefore been adapted in the routine assessment of ALS patients in our centre as well as many others. Although the ECAS has become a standard screening instrument for cognitive assessment in ALS owing to its practicability and feasibility placing far less burden on patients with the shorter testing time, our findings suggest that utilising the ECAS for research purposes is less desirable than in clinical routine.
In conclusion, we found that concurrent cognitive and behavioural impairment in ALS was associated with widespread changes in glucose metabolism. In contrast to previous results which suggested notable differences in glucose hypometabolism between ALSni and ALSci groups, in our study, significant differences were only observed in the group comparison between ALSni and ALScbi. In addition, PVEC did not have a conspicuous effect on FDG-PET analyses in an ALS sample, which suggests that the results from publications with and without PVEC are comparable. The individual cognitive domains did not strongly correlate with glucose metabolism or GM volume, but this result should be replicated in larger samples with more comprehensive cognitive assessments.
Data availability
The data analysed in the current study can be received from the corresponding author upon reasonable request from qualified investigators.
References
Benbrika S, Desgranges B, Eustache F, Viader F (2019) Cognitive, emotional and psychological manifestations in amyotrophic lateral sclerosis at baseline and overtime: a review. Front Neurosci 13:951. https://doi.org/10.3389/fnins.2019.00951
Phukan J, Elamin M, Bede P, Jordan N, Gallagher L, Byrne S, Lynch C, Pender N, Hardiman O (2012) The syndrome of cognitive impairment in amyotrophic lateral sclerosis: a population-based study. J Neurol Neurosurg Psychiatry 83:102–108. https://doi.org/10.1136/jnnp-2011-300188
Beeldman E, Raaphorst J, Klein Twennaar M, de Visser M, Schmand BA, de Haan RJ (2016) The cognitive profile of ALS: a systematic review and meta-analysis update. J Neurol Neurosurg Psychiatry 87:611–619. https://doi.org/10.1136/jnnp-2015-310734
Gibbons ZC, Richardson A, Neary D, Snowden JS (2008) Behaviour in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 9:67–74. https://doi.org/10.1080/17482960701642437
Burke T, Pinto-Grau M, Lonergan K, Bede P, O’Sullivan M, Heverin M, Vajda A, McLaughlin RL, Pender N, Hardiman O (2017) A cross-sectional population-based investigation into behavioral change in amyotrophic lateral sclerosis: subphenotypes, staging, cognitive predictors, and survival. Ann Clin Transl Neurol 4:305–317. https://doi.org/10.1002/acn3.407
Sestini S (2007) Genetic studies of diseases. Cell Mol Life Sci 64:1778–1784. https://doi.org/10.1007/s00018-007-7056-4
Nehlig A, Coles JA (2007) Cellular pathways of energy metabolism in the brain: is glucose used by neurons or astrocytes? Glia 55:1238–1250. https://doi.org/10.1002/glia.20376
Pagani M, Chio A, Valentini MC, Oberg J, Nobili F, Calvo A, Moglia C, Bertuzzo D, Morbelli S, De Carli F, Fania P, Cistaro A (2014) Functional pattern of brain FDG-PET in amyotrophic lateral sclerosis. Neurology 83:1067–1074. https://doi.org/10.1212/WNL.0000000000000792
Van Laere K, Vanhee A, Verschueren J, De Coster L, Driesen A, Dupont P, Robberecht W, Van Damme P (2014) Value of 18 fluorodeoxyglucose–positron-emission tomography in amyotrophic lateral sclerosis: a prospective study. JAMA Neurol 71:553. https://doi.org/10.1001/jamaneurol.2014.62
Buhour M-S, Doidy F, Mondou A, Pélerin A, Carluer L, Eustache F, Viader F, Desgranges B (2017) Voxel-based mapping of grey matter volume and glucose metabolism profiles in amyotrophic lateral sclerosis. EJNMMI Res 7:21. https://doi.org/10.1186/s13550-017-0267-2
Zanovello M, Sorarù G, Campi C, Anglani M, Spimpolo A, Berti S, Bussè C, Mozzetta S, Cagnin A, Cecchin D (2021) Brainstem glucose hypermetabolism in ALS/FTD and shorten survival: a18 F-FDG PET/MR study. J Nucl Med 121:262232. https://doi.org/10.2967/jnumed.121.262232
Jamali AM, Kethamreddy M, Burkett BJ, Port JD, Pandey MK (2023) PET and SPECT imaging of ALS: an educational review. Mol Imaging 2023:1–20. https://doi.org/10.1155/2023/5864391
Canosa A, Moglia C, Manera U, Vasta R, Torrieri MC, Arena V, D’Ovidio F, Palumbo F, Zucchetti JP, Iazzolino B, Peotta L, Calvo A, Pagani M, Chiò A (2021) Metabolic brain changes across different levels of cognitive impairment in ALS: a 18 F-FDG-PET study. J Neurol Neurosurg Psychiatry 92:357–363. https://doi.org/10.1136/jnnp-2020-323876
Carluer L, Mondou A, Buhour M-S, Laisney M, Pélerin A, Eustache F, Viader F, Desgranges B (2015) Neural substrate of cognitive theory of mind impairment in amyotrophic lateral sclerosis. Cortex 65:19–30. https://doi.org/10.1016/j.cortex.2014.12.010
Hinault T, Segobin S, Benbrika S, Carluer L, Doidy F, Eustache F, Viader F, Desgranges B (2022) Longitudinal grey matter and metabolic contributions to cognitive changes in amyotrophic lateral sclerosis. Brain Commun 4:fcac228. https://doi.org/10.1093/braincomms/fcac228
Canosa A, Vacchiano V, D’Ovidio F, Calvo A, Moglia C, Manera U, Vasta R, Liguori R, Arena V, Grassano M, Palumbo F, Peotta L, Iazzolino B, Pagani M, Chiò A (2021) Brain metabolic correlates of apathy in amyotrophic lateral sclerosis: An 18F-FDG-positron emission tomography stud. Eur J Neurol 28:745–753. https://doi.org/10.1111/ene.14637
Hoffman EJ, Huang SC, Phelps ME (1979) Quantitation in positron emission computed tomography: 1. effect of object size. J Comput Assist Tomogr 3:299–308. https://doi.org/10.1097/00004728-197906000-00001
Brooks BR, Miller RG, Swash M, Munsat TL (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1:293–299. https://doi.org/10.1080/146608200300079536
Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, Nakanishi A (1999) The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS study group (Phase III). J Neurol Sci 169:13–21. https://doi.org/10.1016/s0022-510x(99)00210-5
Rascovsky K, Knopman D, Mendez M, Kramer J, Neuhaus J, Swieten J, Seelaar H, Dopper E, Onyike C, Hillis A, Josephs K, Boeve B, Kertesz A, Seeley W, Rankin K, Johnson J, Gorno-Tempini M-L, Rosen H, Miller B (2011) Sensitivity of revised diagnostic criteria for the behavioral variant of frontotemporal dementia. Brain J Neurol 134:2456–2477. https://doi.org/10.1093/brain/awr179
Lulé D, Burkhardt C, Abdulla S, Böhm S, Kollewe K, Uttner I, Abrahams S, Bak T, Petri S, Weber M, Ludolph A (2014) The Edinburgh cognitive and behavioural amyotrophic lateral sclerosis screen: a cross-sectional comparison of established screening tools in a German-Swiss population. Amyotroph Lateral Scler Front Degener. https://doi.org/10.3109/21678421.2014.959451
Stone VE, Baron-Cohen S, Knight RT (1998) Frontal lobe contributions to theory of mind. J Cogn Neurosci 10:640–656. https://doi.org/10.1162/089892998562942
Ekman P, Friesen WV (1975) Unmasking the face: a guide to recognizing emotions from facial clues. Prentice-Hall, Oxford, England
Grace J, Malloy P (2001) FrSBe, frontal systems behavior scale: professional manual. Psychological Assessment Resources, Lutz, FL
Abrahams S, Newton J, Niven E, Foley J, Bak TH (2014) Screening for cognition and behaviour changes in ALS. Amyotroph Lateral Scler Front Degener 15:9–14. https://doi.org/10.3109/21678421.2013.805784
Strong MJ, Abrahams S, Goldstein LH, Woolley S, Mclaughlin P, Snowden J, Mioshi E, Roberts-South A, Benatar M, HortobáGyi T, Rosenfeld J, Silani V, Ince PG, Turner MR (2017) Amyotrophic lateral sclerosis - frontotemporal spectrum disorder (ALS-FTSD): revised diagnostic criteria. Amyotroph Lateral Scler Front Degener 18:153–174. https://doi.org/10.1080/21678421.2016.1267768
Bertoux M, Delavest M, De Souza LC, Funkiewiez A, Lépine J-P, Fossati P, Dubois B, Sarazin M (2012) Social cognition and emotional assessment differentiates frontotemporal dementia from depression. J Neurol Neurosurg Psychiatry 83:411–416. https://doi.org/10.1136/jnnp-2011-301849
Ashburner J (2007) A fast diffeomorphic image registration algorithm. Neuroimage 38:95–113. https://doi.org/10.1016/j.neuroimage.2007.07.007
Müller-Gärtner HW, Links JM, Prince JL, Bryan RN, McVeigh E, Leal JP, Davatzikos C, Frost JJ (1992) Measurement of radiotracer concentration in brain gray matter using positron emission tomography: MRI-based correction for partial volume effects. J Cereb Blood Flow Metab 12:571–583. https://doi.org/10.1038/jcbfm.1992.81
Surti S, Kuhn A, Werner ME, Perkins AE, Kolthammer J, Karp JS (2007) Performance of philips gemini TF PET/CT scanner with special consideration for its time-of-flight imaging capabilities. J Nucl Med 48:471–480
McIntosh AR, Lobaugh NJ (2004) Partial least squares analysis of neuroimaging data: applications and advances. Neuroimage 23:S250–S263. https://doi.org/10.1016/j.neuroimage.2004.07.020
Krishnan A, Williams LJ, McIntosh AR, Abdi H (2011) Partial least squares (PLS) methods for neuroimaging: a tutorial and review. Neuroimage 56:455–475. https://doi.org/10.1016/j.neuroimage.2010.07.034
McHutchison CA, Wuu J, McMillan CT, Rademakers R, Statland J, Wu G, Rampersaud E, Myers J, Hernandez JP, Abrahams S, Benatar M (2023) Temporal course of cognitive and behavioural changes in motor neuron diseases. J Neurol Neurosurg Psychiatry. https://doi.org/10.1136/jnnp-2023-331697
Gainotti G (2019) The role of the right hemisphere in emotional and behavioral disorders of patients with frontotemporal lobar degeneration: an updated review. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2019.00055
Chenji S, Ishaque A, Mah D, Fujiwara E, Beaulieu C, Seres P, Graham SJ, Frayne R, Zinman L, Genge A, Korngut L, Johnston W, Kalra S, for the Canadian ALS Neuroimaging Consortium (CALSNIC) (2021) Neuroanatomical associations of the Edinburgh cognitive and behavioural ALS SCREEN (ECAS). Brain Imaging Behav 15:1641–1654. https://doi.org/10.1007/s11682-020-00359-7
Tjokrowijoto P, Phillips M, Ceslis A, Henderson RD, McCombe PA, Robinson GA (2023) Sensitivity and specificity of the ECAS in identifying executive function and social cognition deficits in MND. Amyotroph Lateral Scler Front Degener. https://doi.org/10.1080/21678421.2023.2188053
Acknowledgements
We thank the patients for taking part in this study.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the professional cyclist André Greipel and his “Fight ALS” initiative; the Boris Canessa ALS Foundation; and the Bundesministerium für Bildung und Forschung/Verein Deutscher Ingenieure (FKZ: 13GW0482D). A.H. is supported by the Hermann und Lilly Schilling Stiftung für medizinsiche Forschung im Stifterverband.
Author information
Authors and Affiliations
Contributions
Manuscript concept and design: AL, ST, JP. Funding acquisition: AH, BJK, JP. Data collection: EK, JK, BJK, JP. Data analysis and interpretation: AL, JS, EK, ST, JP. Manuscript drafting: AL. Manuscript revision and approval of the final version: all.
Corresponding author
Ethics declarations
Conflicts of interest
S.T. served on advisory boards of Lilly, Eisai, and Biogen, and is member of the independent data safety and monitoring board of the ENVISION study (Biogen).
Ethical approval
The study was carried out in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Patients gave their written informed consent, and the local ethics committee at the Rostock University Medical Centre approved the study (2022–0081).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lehto, A., Schumacher, J., Kasper, E. et al. Cerebral glucose metabolic correlates of cognitive and behavioural impairments in amyotrophic lateral sclerosis. J Neurol (2024). https://doi.org/10.1007/s00415-024-12388-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00415-024-12388-z