Abstract
Background
Fatigue is frequent in people with multiple sclerosis (pwMS) impacting physical and cognitive functions. Lower aerobic capacity and regional thalamic volume may be involved in the pathophysiology of fatigue in pwMS.
Objectives
To identify associations between thalamic nuclei volumes, aerobic capacity and fatigue and to investigate whether the influence of aerobic capacity on fatigue in pwMS is mediated by thalamic integrity.
Methods
Eighty-three pwMS underwent a clinical evaluation with assessment of fatigue (Modified Fatigue Impact Scale [MFIS]), including physical (pMFIS) and cognitive (cMFIS) components, and peak of oxygen uptake (VO2peak). PwMS and 63 sex- and age-matched healthy controls (HC) underwent a 3 T brain MRI to quantify volume of the whole thalamus and its nuclei.
Results
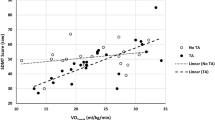
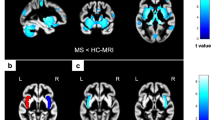
Compared to HC, pwMS showed higher global MFIS, pMFIS and cMFIS scores, and lower VO2peak and thalamic volumes (p < 0.001). In pwMS, higher VO2peak was significantly associated with lower MFIS and pMFIS scores (r value = − 0.326 and − 0.356; pFDR ≤ 0.046) and higher laterodorsal thalamic nucleus (Dor) cluster volume (r value = 0.300; pFDR = 0.047). Moreover, lower Dor thalamic cluster volume was significantly associated with higher MFIS, pMFIS and cMFIS scores (r value range = − 0.305; − 0.293; pFDR ≤ 0.049). The volume of Dor thalamic cluster partially mediated the positive effects of VO2peak on both MFIS and cMFIS, with relative indirect effects of 21% and 32% respectively. No mediation was found for pMFIS.
Conclusions
Higher VO2peak is associated with lower fatigue in pwMS, likely acting on Dor thalamic cluster volume integrity. Such an effect might be different according to the type of fatigue (cognitive or physical).



Similar content being viewed by others
Data availability
The data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Marchesi O et al (2022) Current perspectives on the diagnosis and management of fatigue in multiple sclerosis. Expert Rev Neurother 22(8):681–693
Leocani L, Colombo B, Comi G (2008) Physiopathology of fatigue in multiple sclerosis. Neurol Sci 29(Suppl 2):S241–S243
Capone F et al (2020) Fatigue in multiple sclerosis: the role of thalamus. Mult Scler 26(1):6–16
Kaya Aygünoğlu S et al (2015) Correlation of fatigue with depression, disability level and quality of life in patients with multiple sclerosis. Noro Psikiyatr Ars 52(3):247–251
Vaughn CB et al (2020) Fatigue at enrollment predicts EDSS worsening in the New York State multiple sclerosis consortium. Mult Scler 26(1):99–108
Andreasen AK et al (2010) Fatigue and processing speed are related in multiple sclerosis. Eur J Neurol 17(2):212–218
Mackay L et al (2021) Predictors of cognitive fatigue and fatigability in multiple sclerosis. Mult Scler Relat Disord 56:103316
van Geest Q et al (2018) Information processing speed in multiple sclerosis: Relevance of default mode network dynamics. Neuroimage Clin 19:507–515
Calabrese M et al (2010) Basal ganglia and frontal/parietal cortical atrophy is associated with fatigue in relapsing-remitting multiple sclerosis. Mult Scler 16(10):1220–1228
Bernitsas E et al (2017) Structural and neuronal integrity measures of fatigue severity in multiple sclerosis. Brain Sci. https://doi.org/10.3390/brainsci7080102
Li Y et al (2022) Alterations of thalamic nuclei volumes and the intrinsic thalamic structural network in patients with multiple sclerosis-related fatigue. Brain Sci. https://doi.org/10.3390/brainsci12111538
Preziosa P et al (2023) Structural and functional magnetic resonance imaging correlates of fatigue and dual-task performance in progressive multiple sclerosis. J Neurol 270(3):1543–1563
Hidalgo de la Cruz M et al (2018) Abnormal functional connectivity of thalamic sub-regions contributes to fatigue in multiple sclerosis. Mult Scler 24(9):1183–1195
Strasser B, Burtscher M (2018) Survival of the fittest: VO(2)max, a key predictor of longevity? Front Biosci (Landmark Ed) 23(8):1505–1516
Rooney S et al (2019) Is fatigue associated with aerobic capacity and muscle strength in people with multiple sclerosis: a systematic review and meta-analysis. Arch Phys Med Rehabil 100(11):2193–2204
Mackay CP, Kuys SS, Brauer SG (2017) The effect of aerobic exercise on brain-derived neurotrophic factor in people with neurological disorders: a systematic review and meta-analysis. Neural Plast 2017:4716197
Anderson T, Berry NT, Wideman L (2019) Exercise and the hypothalamic–pituitary–adrenal axis: a special focus on acute cortisol and growth hormone responses. Curr Opin Endocrine Metab Res 9:74–77
Heine M et al (2017) Does aerobic training alleviate fatigue and improve societal participation in patients with multiple sclerosis? A randomized controlled trial. Mult Scler 23(11):1517–1526
Zong B et al (2023) Mechanisms underlying the beneficial effects of physical exercise on multiple sclerosis: focus on immune cells. Front Immunol 14:1260663
Sandroff BM et al (2022) Thalamic atrophy moderates associations among aerobic fitness, cognitive processing speed, and walking endurance in persons with multiple sclerosis. J Neurol 269(10):5531–5540
Petkus AJ et al (2021) Thalamic volume mediates associations between cardiorespiratory fitness (VO(2)max) and cognition in Parkinson’s disease. Parkinsonism Relat Disord. https://doi.org/10.1016/j.parkreldis.2021.03.019
Motl RW et al (2015) Cardiorespiratory fitness and its association with thalamic, hippocampal, and basal ganglia volumes in multiple sclerosis. Neuroimage Clin 7:661–666
Motl RW et al (2021) Do subcortical gray matter volumes and aerobic capacity account for cognitive-motor coupling in multiple sclerosis? Mult Scler 27(3):401–409
Tlamsa AP, Brumberg JC (2010) Organization and morphology of thalamocortical neurons of mouse ventral lateral thalamus. Somatosens Mot Res 27(1):34–43
Ouhaz Z, Fleming H, Mitchell AS (2018) Cognitive functions and neurodevelopmental disorders involving the prefrontal cortex and Mediodorsal thalamus. Front Neurosci. https://doi.org/10.3389/fnins.2018.00033
Homman-Ludiye J, Bourne JA (2019) The medial pulvinar: function, origin and association with neurodevelopmental disorders. J Anat 235(3):507–520
Nelson AJD (2021) The anterior thalamic nuclei and cognition: A role beyond space? Neurosci Biobehav Rev. https://doi.org/10.1016/j.neubiorev.2021.02.047
Motl RW et al (2017) Exercise in patients with multiple sclerosis. Lancet Neurol 16(10):848–856
Morozumi T et al (2023) Influence of cardiorespiratory fitness and MRI measures of neuroinflammation on hippocampal volume in multiple sclerosis. J Neurol Neurosurg Psychiatry 95(1):29–36
Tari AR et al (2019) Are the neuroprotective effects of exercise training systemically mediated? Prog Cardiovasc Dis 62(2):94–101
Fisk JD et al (1994) Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clin Infect Dis 18(Suppl 1):S79-83
Marchesi O et al (2020) Fatigue in multiple sclerosis patients with different clinical phenotypes: a clinical and magnetic resonance imaging study. Eur J Neurol 27(12):2549–2560
Fisk JD et al (1994) The impact of fatigue on patients with multiple sclerosis. Can J Neurol Sci 21(1):9–14
Herdy AH et al (2016) Cardiopulmonary exercise test: background, applicability and interpretation. Arq Bras Cardiol. https://doi.org/10.5935/abc.20160171
van den Akker LE et al (2015) Feasibility and safety of cardiopulmonary exercise testing in multiple sclerosis: a systematic review. Arch Phys Med Rehabil 96(11):2055–2066
Langeskov-Christensen M et al (2014) Validity and reliability of VO2-max measurements in persons with multiple sclerosis. J Neurol Sci 342(1–2):79–87
Valverde S et al (2017) Improving automated multiple sclerosis lesion segmentation with a cascaded 3D convolutional neural network approach. Neuroimage 155:159–168
Iglesias JE et al (2018) A probabilistic atlas of the human thalamic nuclei combining ex vivo MRI and histology. Neuroimage 183(1095–9572):314–326
Weeland CJ et al (2022) The thalamus and its subnuclei—a gateway to obsessive-compulsive disorder. Transl Psychiatry 12(1):70
Field A (2018) Discovering statistics using IBM SPSS statistics, 5th edn. SAGE Publications, London, p 1375
Hair JF et al (2021) Mediation analysis. In: Hair JF et al (eds) Partial least squares structural equation modeling (PLS-SEM) using r: a workbook. Springer International Publishing, Cham, pp 139–153
Moore H et al (2022) Fatigue in multiple sclerosis: a UK MS-register based study. Mult Scler Relat Disord 64:103954
Apollonatou V et al (2023) Cardiopulmonary exercise testing in people with minimally impaired multiple sclerosis. Mult Scler Relat Disord 79:105016
Langeskov-Christensen M et al (2015) Aerobic capacity in persons with multiple sclerosis: a systematic review and meta-analysis. Sports Med 45(6):905–923
Meijboom R et al (2023) Patterns of brain atrophy in recently-diagnosed relapsing-remitting multiple sclerosis. PLoS One 18(7):e0288967
Rocca MA et al (2010) Thalamic damage and long-term progression of disability in multiple sclerosis. Radiology 257(2):463–469
Bezdudnaya T, Keller A (2008) Laterodorsal nucleus of the thalamus: a processor of somatosensory inputs. J Comp Neurol 507(6):1979–1989
Perry BAL, Mitchell AS (2019) Considering the evidence for anterior and Laterodorsal thalamic nuclei as higher order relays to cortex. Front Mol Neurosci 12:167
Romanò F et al (2023) Abnormal thalamic functional connectivity correlates with cardiorespiratory fitness and physical activity in progressive multiple sclerosis. J Neurol 270(6):3213–3224
Chaddock-Heyman L et al (2014) Aerobic fitness is associated with greater white matter integrity in children. Front Hum Neurosci 8:584
Colcombe SJ et al (2006) Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci 61(11):1166–1170
Vakhrusheva J et al (2016) Aerobic exercise in people with schizophrenia: neural and neurocognitive benefits. Curr Behav Neurosci Rep 3(2):165–175
Manjaly ZM et al (2019) Pathophysiological and cognitive mechanisms of fatigue in multiple sclerosis. J Neurol Neurosurg Psychiatry 90(6):642–651
Heesen C et al (2006) Fatigue in multiple sclerosis: an example of cytokine mediated sickness behaviour? J Neurol Neurosurg Psychiatry 77(1):34–39
Consorti A, Di Marco I, Sansevero G (2021) Physical exercise modulates brain physiology through a network of long- and short-range cellular interactions. Front Mol Neurosci. https://doi.org/10.3389/fnmol.2021.710303
Funding
This study has been supported by grant from Italian Ministry of Health (GR-2019–12369599).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
M. Albergoni has nothing to disclose; E. Pagani has nothing to disclose; P. Preziosa received speaker honoraria from Roche, Biogen, Novartis, Merck Serono, Bristol Myers Squibb, Genzyme, Horizon and Sanofi, he has received research support from Italian Ministry of Health and Fondazione Italiana Sclerosi Multipla; A. Meani has nothing to disclose; M.A. Rocca received consulting fees from Biogen, Bristol Myers Squibb, Eli Lilly, Janssen, Roche; and speaker honoraria from AstraZaneca, Biogen, Bristol Myers Squibb, Bromatech, Celgene, Genzyme, Horizon Therapeutics Italy, Merck Serono SpA, Novartis, Roche, Sanofi and Teva. She receives research support from the MS Society of Canada, the Italian Ministry of Health, the Italian Ministry of University and Research, and Fondazione Italiana Sclerosi Multipla. She is Associate Editor for Multiple Sclerosis and Related Disorders. M. Filippi is Editor-in-Chief of the Journal of Neurology, Associate Editor of Human Brain Mapping, Neurological Sciences, and Radiology; received compensation for consulting services from Alexion, Almirall, Biogen, Merck, Novartis, Roche, Sanofi; speaking activities from Bayer, Biogen, Celgene, Chiesi Italia SpA, Eli Lilly, Genzyme, Janssen, Merck-Serono, Neopharmed Gentili, Novartis, Novo Nordisk, Roche, Sanofi, Takeda, and TEVA; participation in Advisory Boards for Alexion, Biogen, Bristol-Myers Squibb, Merck, Novartis, Roche, Sanofi, Sanofi-Aventis, Sanofi-Genzyme, Takeda; scientific direction of educational events for Biogen, Merck, Roche, Celgene, Bristol-Myers Squibb, Lilly, Novartis, Sanofi-Genzyme; he receives research support from Biogen Idec, Merck-Serono, Novartis, Roche, the Italian Ministry of Health, the Italian Ministry of University and Research, and Fondazione Italiana Sclerosi Multipla.
Ethical approval
Approval was received from the institutional ethical standards committee on human experimentation of IRCCS Ospedale San Raffaele (Protocol number 59/INT/2015). Written informed consent was obtained from all subjects prior to study participation according to the Declaration of Helsinki.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Albergoni, M., Pagani, E., Preziosa, P. et al. Thalamic nuclei volume partially mediates the effects of aerobic capacity on fatigue in people with multiple sclerosis. J Neurol (2024). https://doi.org/10.1007/s00415-024-12277-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00415-024-12277-5