Abstract
Background
Neuronal pentraxin-2 (NPTX2), crucial for synaptic functioning, declines in cerebrospinal fluid (CSF) as cognition deteriorates. The variations of CSF NPTX2 across mild cognitive impairment (MCI) due to Alzheimer's disease (AD) and its association with brain metabolism remain elusive, albeit relevant for patient stratification and pathophysiological insights.
Methods
We retrospectively analyzed 49 MCI-AD patients grouped by time until dementia (EMCI, n = 34 progressing within 2 years; LMCI, n = 15 progressing later/stable at follow-up). We analyzed demographic variables, cognitive status (MMSE score), and CSF NPTX2 levels using a commercial ELISA assay in EMCI, LMCI, and a control group of age-/sex-matched individuals with other non-dementing disorders (OND). Using [18F]FDG PET scans for voxel-based analysis, we explored correlations between regional brain metabolism metrics and CSF NPTX2 levels in MCI-AD patients, accounting for age.
Results
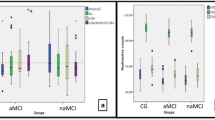
Baseline and follow-up MMSE scores were lower in LMCI than EMCI (p value = 0.006 and p < 0.001). EMCI exhibited significantly higher CSF NPTX2 values than both LMCI (p = 0.028) and OND (p = 0.006). We found a significant positive correlation between NPTX2 values and metabolism of bilateral precuneus in MCI-AD patients (p < 0.005 at voxel level, p < 0.05 with family-wise error correction at the cluster level).
Conclusions
Higher CSF NPTX2 in EMCI compared to controls and LMCI suggests compensatory synaptic responses to initial AD pathology. Disease progression sees these mechanisms overwhelmed, lowering CSF NPTX2 approaching dementia. Positive CSF NPTX2 correlation with precuneus glucose metabolism links to AD-related metabolic changes across MCI course. These findings posit CSF NPTX2 as a promising biomarker for both AD staging and progression risk stratification.



Similar content being viewed by others
Data availability
Data are available on reasonable request from the corresponding author.
References
Colom-Cadena M, Spires-Jones T, Zetterberg H, Blennow K, Caggiano A, DeKosky ST, Fillit H, Harrison JE, Schneider LS, Scheltens P, de Haan W, Grundman M, van Dyck CH, Izzo NJ, Catalano SM (2020) Synaptic Health Endpoints Working Group. The clinical promise of biomarkers of synapse damage or loss in Alzheimer’s disease. Alzheimers Res Ther 12(1):21. https://doi.org/10.1186/s13195-020-00588-4. PMID: 32122400; PMCID: PMC7053087
Sperling RA, Aisen PS, Beckett LA et al (2011) Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dementia 7:280–292. https://doi.org/10.1016/j.jalz.2011.03.003
Lleó A, Núñez-Llaves R, Alcolea D et al (2019) Changes in synaptic proteins precede neurodegeneration markers in preclinical Alzheimer’s disease cerebrospinal fluid*. Mol Cell Proteomics 18:546–560. https://doi.org/10.1074/mcp.RA118.001290
Selkoe DJ (1979) (2002) Alzheimer’s disease is a synaptic failure. Science 298:789–791
Camporesi E, Nilsson J, Brinkmalm A, Becker B, Ashton NJ, Blennow K, Zetterberg H (2020) Fluid Biomarkers for Synaptic Dysfunction and Loss. Biomark Insights 15:1177271920950319. https://doi.org/10.1177/1177271920950319. PMID: 32913390; PMCID: PMC7444114
Milà-Alomà M, Brinkmalm A, Ashton NJ et al (2021) CSF Synaptic Biomarkers in the Preclinical Stage of Alzheimer Disease and Their Association With MRI and PET. Neurology 97:e2065. https://doi.org/10.1212/WNL.0000000000012853
de San G, José N, Massa F, Halbgebauer S et al (2022) Neuronal pentraxins as biomarkers of synaptic activity: from physiological functions to pathological changes in neurodegeneration. J Neural Transm 129:207–230
Llano DA, Devanarayan P, Devanarayan V (2023) CSF peptides from VGF and other markers enhance prediction of MCI to AD progression using the ATN framework. Neurobiol Aging 121:15–27. https://doi.org/10.1016/j.neurobiolaging.2022.07.015
Sathe G, Albert M, Darrow J et al (2021) Quantitative proteomic analysis of the frontal cortex in Alzheimer’s disease. J Neurochem 156:988–1002. https://doi.org/10.1111/jnc.15116
Nilsson J, Gobom J, Sjödin S et al (2021) Cerebrospinal fluid biomarker panel for synaptic dysfunction in alzheimer’s disease. Alzheimer’s Dem Diag Assess Dis Mon. https://doi.org/10.1002/dad2.12179
Libiger O, Shaw LM, Watson MH et al (2021) Longitudinal CSF proteomics identifies NPTX2 as a prognostic biomarker of Alzheimer’s disease. Alzheimer’s Dem 17:1976–1987. https://doi.org/10.1002/alz.12353
Soldan A, Oh S, Ryu T et al (2023) NPTX2 in Cerebrospinal Fluid Predicts the Progression From Normal Cognition to Mild Cognitive Impairment. Ann Neurol. https://doi.org/10.1002/ana.26725
Xiao M-F, Xu D, Craig MT et al (2017) NPTX2 and cognitive dysfunction in Alzheimer’s Disease. Elife. https://doi.org/10.7554/eLife.23798
Watson CM, Dammer EB, Ping L et al (2023) Quantitative Mass Spectrometry Analysis of Cerebrospinal Fluid Protein Biomarkers in Alzheimer’s Disease. Sci Data 10:261. https://doi.org/10.1038/s41597-023-02158-3
Perna A, Marathe S, Dreos R et al (2021) Revealing NOTCH-dependencies in synaptic targets associated with Alzheimer’s disease. Mol Cell Neurosci. https://doi.org/10.1016/j.mcn.2021.103657
Portelius E, Zetterberg H, Skillbäck T et al (2015) Cerebrospinal fluid neurogranin: Relation to cognition and neurodegeneration in Alzheimer’s disease. Brain 138:3373–3385. https://doi.org/10.1093/brain/awv267
Massa F, Halbgebauer S, Barba L et al (2022) Exploring the brain metabolic correlates of process-specific CSF biomarkers in patients with MCI due to Alzheimer’s disease: preliminary data. Neurobiol Aging 117:212–221
Albert MS, DeKosky ST, Dickson D et al (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dem 7:270–279. https://doi.org/10.1016/j.jalz.2011.03.008
Jack CR, Bennett DA, Blennow K et al (2018) NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s and Dementia 14:535–562
Petersen RC, Caracciolo B, Brayne C et al (2014) Mild cognitive impairment: a concept in evolution. J Intern Med 275:214–228. https://doi.org/10.1111/joim.12190
Aisen PS, Petersen RC, Donohue MC et al (2010) Clinical core of the Alzheimer’s disease neuroimaging initiative: Progress and plans. Alzheimer’s and Dementia 6:239–246. https://doi.org/10.1016/j.jalz.2010.03.006
Edmonds EC, McDonald CR, Marshall A et al (2019) Early versus late MCI: Improved MCI staging using a neuropsychological approach. Alzheimer’s Dem 15:699–708. https://doi.org/10.1016/j.jalz.2018.12.009
Soldan A, Moghekar A, Walker KA et al (2019) Resting-state functional connectivity is associated with cerebrospinal fluid levels of the synaptic protein NPTX2 in non-demented older adults. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2019.00132
Galasko D, Xiao M, Xu D et al (2019) Synaptic biomarkers in CSF aid in diagnosis, correlate with cognition and predict progression in MCI and Alzheimer’s disease. Alzheimer’s Dem Translat Res Clin Intervent 5:871–882. https://doi.org/10.1016/j.trci.2019.11.002
Saura CA, Parra-Damas A, Enriquez-Barreto L (2015) Gene expression parallels synaptic excitability and plasticity changes in Alzheimer’s disease. Front Cell Neurosci 9:318. https://doi.org/10.3389/fncel.2015.00318. PMID: 26379494; PMCID: PMC4548151
Duits FH, Brinkmalm G, Teunissen CE et al (2018) Synaptic proteins in CSF as potential novel biomarkers for prognosis in prodromal Alzheimer’s disease. Alzheimers Res Ther. https://doi.org/10.1186/s13195-017-0335-x
DeKosky ST, Scheff SW (1990) Synapse loss in frontal cortex biopsies in Alzheimer’s disease: Correlation with cognitive severity. Ann Neurol 27:457–464
Scheff SW, DeKosky ST, Price DA (1990) Quantitative assessment of cortical synaptic density in Alzheimer’s disease. Neurobiol Aging 11:29–37
Kovács RA, Vadászi H, Bulyáki E et al (2021) Identification of Neuronal Pentraxins as Synaptic Binding Partners of C1q and the Involvement of NP1 in Synaptic Pruning in Adult Mice. Front Immunol. https://doi.org/10.3389/fimmu.2020.599771
Swanson A, Willette AA (2016) Neuronal Pentraxin 2 predicts medial temporal atrophy and memory decline across the Alzheimer’s disease spectrum. Brain Behav Immun 58:201–208. https://doi.org/10.1016/j.bbi.2016.07.148
Zhou J, Wade SD, Graykowski D et al (2023) The neuronal pentraxin Nptx2 regulates complement activity and restrains microglia-mediated synapse loss in neurodegeneration. Sci Transl Med. https://doi.org/10.1126/scitranslmed.adf0141
Nobili F, Schmidt R, Carriò I, Frisoni GB (2018) Brain FDG-PET: clinical use in dementing neurodegenerative conditions. Eur J Nucl Med Mol Imaging 45:1467–1469
Provost K, La Joie R, Strom A et al (2021) Crossed cerebellar diaschisis on 18F-FDG PET: Frequency across neurodegenerative syndromes and association with 11C-PIB and 18F-Flortaucipir. J Cereb Blood Flow Metab 41:2329–2343. https://doi.org/10.1177/0271678X211001216
Zimmer ER, Parent MJ, Souza DG et al (2017) [18F]FDG PET signal is driven by astroglial glutamate transport. Nat Neurosci 20:393–395. https://doi.org/10.1038/nn.4492
Nakashima T, Nakayama N, Miwa K et al (2007) Focal Brain Glucose Hypometabolism in Patients with Neuropsychologic Deficits after Diffuse Axonal Injury. Am J Neuroradiol 28:236
Herholz K (2003) PET studies in dementia. Ann Nucl Med 17:79–89. https://doi.org/10.1007/BF02988444
Soonawala D, Amin T, Ebmeier KP et al (2002) Statistical parametric mapping of 99mTc-HMPAO-SPECT images for the diagnosis of Alzheimer’s disease: Normalizing to cerebellar tracer uptake. Neuroimage 17:1193–1202. https://doi.org/10.1006/nimg.2002.1259
Matsuda H (2001) Cerebral blood flow and metabolic abnormalities in Alzheimer’s disease. Ann Nucl Med 15:85–92. https://doi.org/10.1007/BF02988596
Belbin O, Xiao MF, Xu D et al (2020) Cerebrospinal fluid profile of NPTX2 supports role of Alzheimer’s disease-related inhibitory circuit dysfunction in adults with down syndrome. Mol Neurodegener 15:1–10. https://doi.org/10.1186/s13024-020-00398-0
Acknowledgements
This work was developed within the framework of the DINOGMI Department of Excellence of MIUR 2018-2022 (legge 232 del 2016). The authors are thankful to Davide Visigalli for his technical assistance in the sample pre-analysis and biobanking work.
Funding
This work was partially supported by a grant from the Italian Ministry of Health to IRCCS Ospedale Policlinico San Martino [Fondi per la Ricerca Corrente, and Italian Neuroscience network (RIN)], by #NEXTGENERATIONEU (NGEU) and partially funded by the Ministry of University and Research (MUR), National Recovery and Resilience Plan (NRRP), project MNESYS (PE0000006)—A Multiscale integrated approach to the study of the nervous system in health and disease (DN. 1553 11.10.2022).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
F Massa has received speaker honorarium from Roche Diagnostic Spa, Gómez de San José N is a part-time employer at Proteintech, S Abu-Rumeileh S received research support from the Medical Faculty of Martin-Luther-University Halle-Wittenberg (Clinician Scientist-Program No. CS22/06), D Arnaldi received fees from Fidia, Bruno, Italfarmaco, Idorsia for lectures and board participation; A Uccelli, received grants (to his Institution) from FISM, Biogen, Roche, Alexion, Merck Serono; participated on a Data Safety Monitoring Board or Advisory Board (to his Institution) for BD, Biogen, Iqvia, Sanofi, Roche, Alexion, Bristol Myers Squibb; S Morbelli has received speaker Honoraria from G.E. Healthcare; Otto M received fees for advisory board meetings from Roche, Biogen, Grifols, and Axon; M Pardini receives research support from Novartis and Nutricia, received fees from Novartis, Merck. The other authors have nothing to disclose.
Informed consent and consent to publish
This research involves human participants and was approved by the regional ethical committee (ref:PNRR-POC-2022–12376726). Informed consent was obtained from all participants. Anonymized data published per hospital rules for retrospective data collected during the clinical routine. All procedures followed the 1964 Helsinki Declaration and its later amendments.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Massa, F., Martinuzzo, C., Gómez de San José, N. et al. Cerebrospinal fluid NPTX2 changes and relationship with regional brain metabolism metrics across mild cognitive impairment due to Alzheimer's disease. J Neurol 271, 1999–2009 (2024). https://doi.org/10.1007/s00415-023-12154-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-12154-7