Abstract
Importance
Early treatment initiation in multiple sclerosis (MS) is crucial in preventing irreversible neurological damage and disability progression. The current assessment of disease activity relies on relapse rates and magnetic resonance imaging (MRI) lesion activity, but inclusion of other early, often “hidden,” indicators of disease activity may describe a more comprehensive picture of MS.
Observations
Early indicators of MS disease activity other than relapses and MRI activity, such as cognitive impairment, brain atrophy, and fatigue, are not typically captured by routine disease monitoring. Furthermore, silent progression (neurological decline not clearly captured by standard methods) may occur undetected by relapse and MRI lesion activity monitoring. Consequently, patients considered to have no disease activity actually may have worsening disease, suggesting a need to revise MS management strategies with respect to timely initiation and escalation of disease-modifying therapy (DMT). Traditionally, first-line MS treatment starts with low- or moderate-efficacy therapies, before escalating to high-efficacy therapies (HETs) after evidence of breakthrough disease activity. However, multiple observational studies have shown that early initiation of HETs can prevent or reduce disability progression. Ongoing randomized clinical trials are comparing escalation and early HET approaches.
Conclusions and relevance
There is an urgent need to reassess how MS disease activity and worsening are measured. A greater awareness of “hidden” indicators, potentially combined with biomarkers to reveal silent disease activity and neurodegeneration underlying MS, would provide a more complete picture of MS and allow for timely therapeutic intervention with HET or switching DMTs to address suboptimal treatment responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is a chronic, heterogenous, inflammatory and neurogenerative disease, characterized by demyelinating lesions in the central nervous system. MS can be categorized as clinically isolated syndrome (CIS), relapsing–remitting MS (RRMS), primary progressive MS, and secondary progressive MS (SPMS) [1]. An early asymptomatic phase with brain magnetic resonance imaging (MRI) abnormalities suggestive of MS has been termed radiologically isolated syndrome (RIS) [2].
Effective control of MS disease activity initiated early in the disease course, before MS-related nervous system damage becomes irreversible, is critical. Early and accurate MS diagnosis allows for early intervention to optimize long-term patient outcomes [3, 4]. This is particularly relevant in children and younger adults in whom treatment effects are amplified and have greater success in slowing progression, when compared with older patients [5, 6].
Currently, clinical guidelines define MS activity as demonstration of relapses or radiologic activity, which are measured by new or active lesions on MRI [7, 8]. However, relapses and MRI lesions largely reflect only the focal inflammatory aspects of disease activity; the accrual of disability, manifested through worsening on neurological examination, and more subtle signs such as fatigue or impaired cognition, may be missed. Furthermore, an apparent clinical–radiologic paradox exists, where clinical and radiologic evidence can be poorly correlated [9]. Advanced imaging, nonimaging and soluble biomarkers, such as neurofilament light chain (NfL) levels, may provide more sensitive measures of underlying disease activity that could help explain the clinical–radiologic paradox. Measures of disease activity that capture inflammatory, neurodegenerative, and disability elements may allow faster identification of underlying disease activity and suboptimal response to therapy, thus improving MS risk assessment and treatment decision making.
The implementation of high-efficacy therapies (HETs) as first-line treatment may delay disability progression and improve clinical outcomes [3, 10]. However, the current guidelines recommend HET use in patients with highly active MS [7, 11, 12]. Moreover, definitions of highly active disease have differed across clinical trials and include the occurrence of clinical relapses and lesion activity as detected by MRI [7], which may not fully capture disease activity.
This review explores early, often “hidden” indicators of disease activity, such as changes in cognition and fatigue and biomarkers of disease activity, to support characterization of a more comprehensive picture of MS, and which should be considered in the management of confirmed early MS. We also present current evidence regarding the early use of HETs and the unmet need for treatment guidelines that include early MS activity and recommendations regarding prompt HET intervention.
Current definitions of disease activity
MS diagnosis
MS is diagnosed according to the most recent version of the McDonald criteria [13]. This requires confirmation of central nervous system (CNS) disease disseminated in time and space as demonstrated by clinical attacks, examination features, MRI, and cerebrospinal fluid (CSF) analysis [13]. The McDonald criteria and its subsequent revisions have helped to decrease delays in diagnosis, notably among children, adolescents, and younger adults [14,15,16]. Clinical relapses and MRI disease activity are prioritized in the most recent criteria [13].
Although the 2017 updates to the McDonald criteria aimed to provide guidance to avoid the misdiagnosis of MS [13], it is important to acknowledge that misdiagnosis remains a concern in clinical practice [17, 18]. One 2019 study found that approximately 1 in 5 patients with a diagnosis of MS did not actually meet the diagnosis criteria upon reevaluation at an MS subspecialty center [17]. The proper application of the McDonald criteria is critical for a correct diagnosis of MS, as patients receiving a misdiagnosis of MS may be exposed to unnecessary risks when incorrectly prescribed DMTs and may incur considerable financial burden [17].
Disease activity in clinical guidelines
Practice guidelines from the American Academy of Neurology measure disease activity by clinical relapses or new MRI lesions, and these assessments are used to guide MS monitoring and treatment [7]. Similarly, treatment guidelines from the European Committee of Treatment and Research in MS and the European Academy of Neurology describe disease activity in patients with relapsing forms of MS in terms of relapses, disability progression, and MRI activity [11].
Early disability accrual in MS
There is considerable evidence that, in the early stages of MS, pathological changes occur that are not reflected in relapses and MRI lesions. “Hidden” pathological changes such as decreased cognitive performance, anxiety, depression, and migraine, have been detected years before typical MS symptoms appear and have been identified as prodromal features in MS (Fig. 1) [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40]. In addition, the disease progression can occur independently of relapses in patients with RRMS, termed progression independent of relapse activity (PIRA) [41, 42]. In phase 3 trials in recently diagnosed treatment-naïve patients with relapsing MS, over half of confirmed disability worsening events occurred due to PIRA [43].
Subclinical or “hidden” indicators of disease activity, identified from prediagnosis of MS through early RRMS, that represent disease activity. CIS, clinically isolated syndrome, GFAP glial fibrillary acidic protein, IgG, immunoglobulin G, MS multiple sclerosis, NfL neurofilament light, RIS radiologically isolated syndrome, RRMS relapsing–remitting multiple sclerosis
Impaired cognition
Cognitive impairment is a core symptom of MS; patients consider nonphysical MS symptoms such as cognitive impairment as having a substantial impact on their quality of life [30, 44]. Guidelines for identifying and monitoring cognitive changes in MS were published in 2018 by the National Multiple Sclerosis Society, but do not discuss prognostic applications [30].
Cognitive impairment has been detected in patients with RIS and may be consistent with a diagnosis of subclinical MS [24]; cognitive impairment in CIS may actually predict conversion to MS [45]. Cognitive impairment can be common in early MS [40, 46]: in a study of 92 patients with RRMS with very mild or no clinical disability, half had cognitive impairment (across five tools measuring verbal and visuospatial long-term memory, information processing speed, and executive functions) [47]. Conversely, a study in patients with RRMS (N = 128) found impairments in phonemic fluency in patients with early RRMS (< 3 years) versus healthy controls (N = 63), but other assessments did not differ [48]. Regarding children with MS, there is disagreement on whether changes in academic performance indicate MS prodromal cognitive decline; one study identified a potential link [49], whereas another did not [50].
The importance of detecting these early cognitive changes is apparent from a study in > 1000 patients with MS that showed irreversible axonal damage and brain tissue loss accumulation before cognitive decline became evident [51]. Cognitive impairment has also been linked to CSF molecular patterns showing innate and adaptive immune responses in newly diagnosed patients [52] and gray matter damage [53]. Although cognitive impairment may go unnoticed in early MS due to compensatory neurological mechanisms [54], early control of inflammation could prevent or limit cognitive impairment. Moreover, changes in cognition could indicate suboptimal MS disease control and underlying disease activity and progression and therefore signify an urgency to initiate or change MS treatment [30, 45].
A unified set of criteria for identifying cognitive impairment early in MS is needed [55]. Several validated assessments already exist and are summarized in Table 1 [30, 56,57,58]. Because no single tool is currently widely used, it is reasonable to adopt an easy-to-use tool not requiring psychological training to administer in clinical practice. However, such a tool should be validated to the larger, more complete neuropsychological battery to establish if the results are valid and if it is a useful surrogate for the more time-consuming neuropsychological battery. Additional concerns include learning effects on repeated administration, and the usefulness of repeated testing to detect cognitive decline using the same instrument has been questioned [59].
Neuropsychiatric symptoms
Neuropsychiatric symptoms, including mild depressive symptoms and fatigue, have been reported in patients with CIS who did not meet the diagnostic MRI criteria for MS; anxiety and depression were associated with lower normal white matter volume and higher lesion load on MRI, respectively [28]. Patients can also experience sleep disorders, anxiety, depression, and migraine both before their MS diagnosis and after MS is diagnosed [3, 21, 34, 36, 38, 60, 61].
In patients with MS, fatigue is among the most common symptoms and substantially impacts quality of life [3]. Fatigue and depression are linked to cognitive impairment; fatigue has also been associated with brain lesions and both CNS and peripheral inflammation [62, 63]. Therefore, fatigue may be associated with underlying neurodegeneration or inflammation.
Currently, symptoms such as fatigue are not well captured by the Expanded Disability Status Scale (EDSS) [3], nor in the current definitions of disease activity. In addition, neuropsychiatric signs and symptoms can be the initial presenting complaint ahead of a definitive MS diagnosis [64], but these are not captured in the McDonald criteria [65, 66]. A retrospective study of 281 referrals to an MS center for a question of MS diagnosis reported that patients with only vague, nonspecific symptoms (such as headache) were not diagnosed with MS [65]. Another study reported that 96 of 244 referrals for a new diagnosis of MS presented with atypical symptoms, of whom only 15 were diagnosed with MS or CIS [67]. As such, patients exhibiting these symptoms currently have no pathway to reach a diagnosis until a clinical attack occurs or there is clear progression of neurological disability. Nonetheless, the Beck Depression Inventory—Fast Screen, the Hospital Anxiety and Depression Scale, and the Patient Health Questionnaire-9 are suggested for evaluating depression and anxiety in adults with MS [30, 68]. Monitoring children with MS for behavioral and academic performance changes is also recommended [30].
As the clinical significance of changes in these symptoms during early MS becomes more defined, updated disease management strategies may be required. For instance, clinicians’ discussions with patients and their caregivers provide opportunities to identify the first signs of “hidden” symptoms, thereby enabling earlier intervention.
Biomarkers as indicators of “silent” disease
Although there is currently no laboratory test to diagnose MS in the absence of clinical or imaging findings [13], several molecules are elevated in the blood or CSF of patients with MS that could have the potential to be prognostic markers. However, all biomarkers described in the following sections are still in investigational stages.
Molecular markers: focus on immunoglobulins
Oligoclonal bands (OCBs) of immunoglobulin G (IgG) are present in the CSF in up to 90% of patients with MS [26]. The presence of OCBs early in the MS disease course correlates with relapses and is associated with disease progression [26, 69, 70]. Although OCBs already form part of the McDonald diagnostic criteria [13], further confirmatory studies are required to establish OCB as a prognostic marker [71]. The IgG index is widely used as a diagnostic marker of MS and is based on elevated IgG levels in the CSF relative to the reference protein albumin; however, it can lack the sensitivity of OCBs and be influenced by age and fluctuations in albumin levels [71, 72].
Molecular markers: focus on NfL
Multiple studies have reported elevated NfL levels in the peripheral blood and CSF of patients with MS [35, 73]. Neurofilaments are neuron-specific cytoskeletal proteins located within myelinated CNS and peripheral nervous system neurons that are released into the surrounding milieu upon neuronal damage [74, 75] (e.g., as a result of acute inflammation-mediated axonal damage [76]). NfL has been evaluated extensively in MS (Table 2) [20, 73, 77,78,79,80,81,82,83] and shows great potential as a biomarker in monitoring disease activity in MS [73].
The 2021 Consortium of Multiple Sclerosis Centers (CMSC) Consensus Statement on Neurofilament Biomarkers in MS highlighted potential applications for NfL measurements for clinical decision-making during the course of MS, adding that serum and/or CSF NfL could complement MRI monitoring for detecting underlying inflammatory activity and informing risk of future MS disease burden [84].
There is a growing body of evidence to support NfL as a prognostic marker in early MS because it can predict MRI lesions, brain atrophy, and disability progression up to 10 years in advance (Table 2) [79,80,81,82,83, 85]. However, unresolved questions around cutoff values for NfL concentration and standardization of NfL measurement currently preclude its use as a biomarker in clinical practice [86]. In addition, the prognostic value of NfL level in progressive forms of MS is less clear due to the current knowledge gaps [84].
Understanding the confounding factors that can affect NfL levels, such as age, obesity, diabetes, kidney function, and certain types of drugs [84, 87], as well as assay standardization, will facilitate clinical implementation of this biomarker [84]. In addition, the use of NfL for diagnosis in real-world clinical practice requires normative NfL data with which to interpret baseline serum levels and to define clinically meaningful changes [84].
Molecular markers: focus on glial fibrillary acidic protein (GFAP)
GFAP levels are elevated in the CSF of patients with MS [35]. GFAP has been explored as a marker of astrocyte damage and loss, which could predict disease severity, progression, and activity in MS (Table 2) [32, 88]. However, to date, there is no published evidence supporting GFAP as a marker for subclinical worsening in early MS. In addition, some of the limitations of NfL in real-world clinical practice likely apply to GFAP; for example, confounding factors affecting serum GFAP levels need to be identified and normative GFAP data are required before this marker can be used in clinical practice.
Other molecular markers
Other molecules elevated in patients with MS include total tau protein (t-tau), chitinase-3-like protein 1 (CHI3L1), and S100B; t-tau and CHI3L1 are also elevated in those with CIS [35]. These biomarkers could potentially be used in the diagnosis and/or to prognosticate risk of MS. A meta-analysis in 338 patients reported decreased levels of brain-derived neurotrophic factor (BDNF) levels in the blood of patients with MS [89]. BDNF levels in the CSF at the time of MS diagnosis were inversely associated with cognitive performance in one study; therefore, the authors proposed that BDNF in combination with NfL could be a potential biomarker for impaired cognition in MS but recognize that integration of these measurements into clinical practice could be challenging [90]. Further research is needed to fully understand the role of these markers in the disease course [35, 90].
MRI-based biomarkers in early MS
Brain atrophy as detected by MRI is estimated to occur at ~ 0.1–0.3% per annum as part of the normal aging process; however, age-dependent atrophy occurs more rapidly at an annual rate of 0.5–1.3% in untreated MS [91, 92]. The neurologic reserve represents the capacity of the CNS to compensate for injury through remodeling [3]; it has been proposed that brain atrophy in patients with subclinical MS depletes the neurologic reserve to a point where the brain can no longer compensate for MS-related damage, after which clinical symptoms become apparent and the disease progresses [3]. In fact, a cohort study in 140 patients with MS revealed that the rate of brain atrophy was highest during the first 5 years of the disease, especially in younger patients [93]; furthermore, early brain atrophy may be associated with early focal lesion accumulation [94] and with higher fatigue and cognitive impairment [95]. Although the tools for measuring brain atrophy at the patient level in clinical practice have been recently described, longitudinal studies to assess brain atrophy changes over time are required to test the reliability of these MRI tools [95]. Consistency between clinical MRI scans over time can also be difficult to achieve [96]. Nevertheless, early treatment with DMTs could prevent this accelerated damage to the CNS [3].
Paramagnetic rim lesions (PRLs), also known as iron rim lesions (IRLs), are thought to reflect chronic active lesions with substantial microglia/macrophage inflammation [97, 98]. Retrospective studies of patients with MS have found that, compared with patients without PRLs, patients with least one PRL had higher disability scores, T2 lesion volume, and intrathecal IgG synthesis were higher, and lower brain volume, and patients with 4 or more PRLs had more aggressive disease, and experienced greater motor and cognitive disability at an earlier age [97, 98]. Also, PRLs have been detected in early RRMS [99]. PRLs represent MRI biomarkers that may reflect more compartmentalized inflammation [97] as they can be tracked over time and have promise in clinical trials to assess treatment response [100].
Slowly expanding lesions (SELs) are another proposed biomarker of chronic lesion activity [101]. Although SELs are more frequently thought to be associated with progressive MS [102], they have been reported to develop in 92% of patients with early RRMS [101]. Moreover, a higher number, volume and relative proportion of SELs—and in combination with PRLs – have been associated with a higher risk of disability progression and conversion to SPMS when identified in patients with early RRMS [101, 103]. In fact, SELs co-localized with PRLs, which exhibit severe accumulation of active tissue damage over time, may represent the most destructive type of chronic MS lesion [104]. As such, the presence of SELs and PRLs could serve as a biomarker and predictor of more severe disease activity early in the disease course [101, 103]. However, PRL validation and reliability, standardization of MRI methods, and clinical training of neurologists and neuroradiologists are required before PRLs can be implemented in clinical practice [105]. It is likely that additional technical requirements will also apply to the use of SELs in clinical practice.
MRI can also be used to detect veins located in the center of white matter lesions; this “central vein sign” can predict a diagnosis of MS in patients with otherwise atypical features of MS. With further research, this potential biomarker could facilitate diagnosis in clinical practice [106].
Both PRLs and the central vein sign appear to be specific for MS and have shown specificity for MS comparable and in some cases greater than OCBs: one study of scans from 112 patients with CIS and 35 in a non-MS group taken in routine clinical assessments found that the presence of ≥ 3 lesions with central vein signs or one PRL had high sensitivity (70%) and specificity (86%) for predicting conversion from CIS to MS [107]. Similarly, a retrospective study of 412 cases revealed that central vein signs could discriminate MS from non-MS cases with 99% sensitivity and 96% specificity; a combination of ≥ 1 PRL and ≥ 40% central vein signs gave a sensitivity of 59% and specificity of 99% [108]. Used in combination, these markers could be highly useful in the differential diagnosis of MS, but more prospective studies are required to validate PRLs and central vein signs as diagnostic biomarkers [108].
Disease-modifying therapies (DMTs)
Clinical guidelines on the use of disease-modifying therapies
Early treatment with DMTs is recommended for those patients with active RRMS or CIS, as defined by clinical relapses and/or MRI activity (active lesions, new or enlarging T2 lesions) [11]. In many cases, HETs are reserved for patients with highly active MS, which may be based on relapses and MRI activity [7], although it is becoming an increasingly common practice in certain MS centers and clinical practices to use HET even in the absence of classical markers of highly active disease [109, 110]. Current MS guidelines do not include management of RIS [7, 11]; however, a recent clinical trial showed that DMT use in patients with RIS could significantly reduce the risk of a first clinical demyelinating event and reduce the number of MRI lesions [111], which supports early treatment in the spectrum of demyelinating disease [111]. Furthermore, a recent observational study of 580 patients with a first demyelinating event reported an association between early treatment and a reduction in the long-term risk of disability accrual [112]. The results from other ongoing clinical trials investigating DMTs in patients with RIS could inform the treatment of these patients [113, 114].
Early initiation of HET
Currently approved HETs include alemtuzumab, cladribine, natalizumab, ocrelizumab, ofatumumab, and ublituximab [115, 116]; opinion is mixed on the exact definition of HETs and whether sphingosine-1-phosphate modulators (fingolimod, siponimod, ozanimod, and ponesimod) should be included (Table 3) [117].
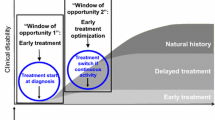
A considerable body of evidence exists showing improved outcomes for patients with MS with early HET versus early medium-efficacy therapy and for early versus delayed use of HET, including delays in cognitive worsening and reductions in disability progression, relapses rates, and MRI activity (Table 3) [10, 118,119,120,121,122,123,124,125]. Moreover, the window of opportunity for optimum benefit from treating MS with HET is in the early stages of disease and at younger age [126]; a meta-analysis of 38 studies in > 28,000 subjects demonstrated an advantage for HET over low-efficacy therapy in early MS, before ~ 40 years of age [127]. The detection of PIRA and “silent progression” in patients with early MS, along with clinical evidence of HETs preventing disability accumulation regardless of relapse, further supports the use of early HET [41, 43, 128, 129].
Two randomized clinical trials, Traditional Versus Early Aggressive Therapy for Multiple Sclerosis (TREAT-MS; NCT03500328) and Determining the Effectiveness of early Intensive Versus Escalation Approaches for RRMS (DELIVER-MS, NCT03535298), are currently underway to examine the utility of early HET [130, 131]. With an estimated completion date of August 2025, TREAT-MS is a pragmatic controlled trial to evaluate whether early HET (natalizumab, alemtuzumab, ocrelizumab, rituximab, cladribine, or ofatumumab) versus traditional first-line therapy affects disability risk, and the effects on disability risk of switching to HET after breakthrough disease [131]. DELIVER-MS, with an estimated completion date of September 2026, is investigating whether early treatment with HET (alemtuzumab, natalizumab, rituximab, or ocrelizumab) improves prognosis by measuring brain atrophy over 3 years [130, 132]. It is anticipated that these trials will help guide treatment paradigms with existing and new therapies and support treatment decision making [130]. Furthermore, these trials could provide insights into brain atrophy, cognitive function, and patient-reported outcomes with early HET.
Although HET initiation early in the disease course may improve prognosis, several barriers to early HET versus escalation therapy exist. Fewer than 25% of patients diagnosed with MS received HET as their first-line treatment in Europe in 2019; HET is also substantially underused in the United States [115, 133]. A current lack of long-term randomized controlled trials informing on this approach means that payers and physicians could be reticent in using early HET until robust evidence exists. As such, national and regional guidelines still recommend starting treatment with less-effective therapy and restrict HET to later stages or after treatment failure; these delays can be due to reimbursement rather than regulatory criteria [115, 133]. Similarly, insurance companies can restrict access to certain DMTs, including HETs, despite their regulatory approval [134]. Among physicians, hesitancy in prescribing HETs may be due to safety concerns associated with more potent anti-inflammatory agents as well as reduced urgency and consideration for factors associated with poor prognosis in MS [115, 133]. In addition, the management of HET initiation and monitoring can present a challenge [135].
The benefit–risk profile for early use of HET should be considered in individual patients with an MS diagnosis and weighed against the risk of MS disease progression; decision-making needs to consider risks associated with specific HETs [115]. Therefore, patients need to be involved in treatment decisions so their preferences and concerns are addressed [7], because patients and physicians may not have the same perceptions of treatment risk [136]. In addition, it is anticipated that DELIVER-MS and TREAT-MS will shed further light on the evolving paradigm of HET for early MS in the real-world setting.
The accumulating evidence in support of early HET creates an urgency that may put pressure on neurologists to diagnose MS early, which could increase the risk of misdiagnosis if the McDonald criteria have not been correctly applied [66]. It is therefore paramount that an accurate diagnosis is made in line with the McDonald criteria to ensure optimal outcomes for patients [66, 137].
Disease activity after DMT initiation
After initiation of DMTs, patients may be classified based on the presence of relapses and MRI lesion activity. However, phase 3 clinical trials have revealed a considerable proportion of patients with PIRA after treatment with DMTs [43, 128]. In addition, an observational study in 480 patients with CIS or RRMS found that long-term disease evolution to SPMS occurred independently of relapses and new MRI lesions [129]. Hidden indicators of MS, such as cognitive impairment, depression, anxiety, fatigue, and sleep disturbance, did not significantly improve with DMT use in an observational study of 440 patients [138]. Patients with MS have reported that these hidden indicators are not sufficiently prioritized and addressed with currently available therapies; thus, a more comprehensive treatment approach is required [139]. Taken together, these observations highlight the need to more fully assess the effectiveness of existing DMTs in mitigating factors of disease beyond relapses and lesion activity.
In addition to their use in early disease, molecular biomarkers could help monitor disease progression after DMT initiation in the absence of relapses or MRI lesion activity. The CMSC has proposed the use of CSF and serum NfL in determining response to therapy at an individual level [84]. In addition, NfL could be used to measure disease activity beyond that detected with clinical and MRI markers, and as a biomarker to monitor treatment effectiveness (Table 2). However, more evidence is needed to determine the role of molecular biomarkers in the decision to escalate treatment from a traditional therapy to a HET.
No evidence of disease activity (NEDA) is a marker used in clinical trials to measure disease activity and treatment response; NEDA-3 is a composite of no relapses, no disability progression, and no MRI lesion activity [140]. However, focusing solely on these measures risks missing underlying neurodegeneration and brain atrophy, often termed “silent progression” [129]. In one study of 42 patients with MS, although 31% achieved NEDA-3 after 2 years, 58% of those with NEDA-3 still developed cognitive worsening [141]. In addition, a longitudinal registry study found that NEDA-3 at 2 years did not predict long-term disease stability [142]. Consequently, a more stringent NEDA-4 is used, which includes additional measures of disease activity, such as brain atrophy [143], NfL [144, 145], or Symbol Digit Modalities Test (SDMT) [146]. Multiple studies with DMTs have demonstrated the greater stringency of NEDA-4 over NEDA-3; a pooled analysis of clinical trials found that 31.0% of 783 fingolimod-treated patients achieved NEDA-3, whereas only 19.7% of 706 patients achieved NEDA-4 (NEDA-3 plus brain atrophy) [143]. Similarly, a phase 3 trial of ponesimod reported 25.0% of patients achieved NEDA-3, but only 11.4% achieved NEDA-4 (NEDA-3 plus brain atrophy) [147]. In an observational study, 58% of 48 patients achieved NEDA-3, whereas only 29% achieved NEDA-4 (NEDA-3 plus brain atrophy) [148]. Another observational study of 45 patients reported 1-year NEDA rates of 60% for NEDA-3, 38% for NEDA-4 (NEDA-3 plus brain atrophy), and 53% for NEDA-4 (NEDA-3 plus SDMT) [146]. An expanded definition of NEDA (NEDA-5) that includes cognition, brain atrophy, inflammatory, and axonal damage markers has been proposed [149]. This would further lower the threshold to detect potentially clinically meaningful changes in patients, to promote urgency in clinical decision-making for patients treated suboptimally.
Consensus guidelines for identifying and managing early disease activity: an unmet need
Greater emphasis on “hidden” symptoms of early MS, such as cognitive and other neuropsychiatric changes, allows disability accrual to be measured more completely and warrants further consideration for future diagnostic and monitoring guidelines. Validated assessment tools for cognitive testing, combined with unified criteria for identifying cognitive impairment, would enable this approach. Electronic self-administered tools such as the Processing Speed Test are potentially practical and convenient ways of testing cognition. In the near future, as validated assays and data demonstrating actionable thresholds become available, biomarkers such as NfL could be used in real-world clinical practice for prognosis alongside clinical evaluations and imaging [84].
In the future, new consensus will likely be needed on early use of HETs as new data on “hidden” symptoms become available. The 2017 update of the McDonald criteria advises caution and postponement of a definitive MS diagnosis and delayed initiation of long-term DMTs in cases presenting atypical features or in the absence of typical CIS, to avoid misdiagnosis [13]. This highlights the importance of diagnostic tools and markers in atypical cases to enable prompt and accurate diagnosis, and therefore early initiation of therapy.
Judicious guidelines on starting therapy that take prognostic factors into consideration are required to ensure patients receive effective therapy and provide budget certainty to payers [7, 115]. Any concerns around initiating early HETs must be weighed against the risk to patients of disease progression and irreversible brain damage through missed opportunities of early effective treatment [10, 115]. A comprehensive therapeutic algorithm for treatment decisions may be required to improve access to HET [115]; this could integrate a range of prognostic factors to enable better risk stratification [115, 150].
Conclusions
Diagnosis and treatment of MS at the earliest possible stages are critical for optimal outcomes. Although current guidelines define disease activity primarily in terms of clinical relapses and MRI lesion activity, these measures do not necessarily capture early pathological changes in MS. Impaired cognition, fatigue, brain atrophy, and elevated NfL levels are evident early in the disease process and may be used as indicators of poor prognosis. Therefore, these should be considered when defining disease activity, to provide a more integrated and comprehensive understanding of MS that reflects underlying neurodegeneration, other disease signs, and patient experiences.
Early detection of MS allows early treatment initiation. In light of emerging evidence and pending outcomes of ongoing clinical trials, early HET is appropriate and can be considered not only in cases of severe disease activity. Long-term safety data and clinical trial data on early HET are needed to help give physicians and payers confidence in its use. As new data from ongoing clinical trials become available, the current management guidelines will require updating to provide additional support to physicians with regard to early initiation of HET.
Availability of data and materials
Not applicable.
References
Klineova S, Lublin FD (2018) Clinical course of multiple sclerosis. Cold Spring Harb Perspect Med. https://doi.org/10.1101/cshperspect.a028928
Filippi M, Bar-Or A, Piehl F, Preziosa P, Solari A, Vukusic S, Rocca MA (2018) Multiple sclerosis. Nat Rev Dis Primers 4:43. https://doi.org/10.1038/s41572-018-0041-4
Giovannoni G, Butzkueven H, Dhib-Jalbut S, Hobart J, Kobelt G, Pepper G, Sormani MP, Thalheim C, Traboulsee A, Vollmer T (2016) Brain health: time matters in multiple sclerosis. Mult Scler Relat Disord 9(suppl-1):S5–S48. https://doi.org/10.1016/j.msard.2016.07.003
Thrue C, Riemenschneider M, Hvid LG, Stenager E, Dalgas U (2021) Time matters: early-phase multiple sclerosis is accompanied by considerable impairments across multiple domains. Mult Scler 27:1477–1485. https://doi.org/10.1177/1352458520936231
Signori A, Schiavetti I, Gallo F, Sormani MP (2015) Subgroups of multiple sclerosis patients with larger treatment benefits: a meta-analysis of randomized trials. Eur J Neurol 22:960–966. https://doi.org/10.1111/ene.12690
Baroncini D, Simone M, Iaffaldano P, Brescia Morra V, Lanzillo R, Filippi M, Romeo M, Patti F, Chisari CG, Cocco E, Fenu G, Salemi G, Ragonese P, Inglese M, Cellerino M, Margari L, Comi G, Zaffaroni M, Ghezzi A (2021) Risk of persistent disability in patients with pediatric-onset multiple sclerosis. JAMA Neurol 78:726–735. https://doi.org/10.1001/jamaneurol.2021.1008
Rae-Grant A, Day GS, Marrie RA, Rabinstein A, Cree BAC, Gronseth GS, Haboubi M, Halper J, Hosey JP, Jones DE, Lisak R, Pelletier D, Potrebic S, Sitcov C, Sommers R, Stachowiak J, Getchius TSD, Merillat SA, Pringsheim T (2018) Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 90:777–788. https://doi.org/10.1212/wnl.0000000000005347
Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sorensen PS, Thompson AJ, Wolinsky JS, Balcer LJ, Banwell B, Barkhof F, Bebo B Jr, Calabresi PA, Clanet M, Comi G, Fox RJ, Freedman MS, Goodman AD, Inglese M, Kappos L, Kieseier BC, Lincoln JA, Lubetzki C, Miller AE, Montalban X, O’Connor PW, Petkau J, Pozzilli C, Rudick RA, Sormani MP, Stuve O, Waubant E, Polman CH (2014) Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 83:278–286. https://doi.org/10.1212/WNL.0000000000000560
Barkhof F (2002) The clinico-radiological paradox in multiple sclerosis revisited. Curr Opin Neurol 15:239–245. https://doi.org/10.1097/00019052-200206000-00003
Simonsen CS, Flemmen HØ, Broch L, Brunborg C, Berg-Hansen P, Moen SM, Celius EG (2021) Early high efficacy treatment in multiple sclerosis is the best predictor of future disease activity over 1 and 2 years in a Norwegian population-based registry. Front Neurol 12:693017. https://doi.org/10.3389/fneur.2021.693017
Montalban X, Gold R, Thompson AJ, Otero-Romero S, Amato MP, Chandraratna D, Clanet M, Comi G, Derfuss T, Fazekas F, Hartung HP, Havrdova E, Hemmer B, Kappos L, Liblau R, Lubetzki C, Marcus E, Miller DH, Olsson T, Pilling S, Selmaj K, Siva A, Sorensen PS, Sormani MP, Thalheim C, Wiendl H, Zipp F (2018) ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Eur J Neurol 25:215–237. https://doi.org/10.1111/ene.13536
Scolding N, Barnes D, Cader S, Chataway J, Chaudhuri A, Coles A, Giovannoni G, Miller D, Rashid W, Schmierer K, Shehu A, Silber E, Young C, Zajicek J (2015) Association of British Neurologists: revised (2015) guidelines for prescribing disease-modifying treatments in multiple sclerosis. Pract Neurol 15:273–279. https://doi.org/10.1136/practneurol-2015-001139
Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, Correale J, Fazekas F, Filippi M, Freedman MS, Fujihara K, Galetta SL, Hartung HP, Kappos L, Lublin FD, Marrie RA, Miller AE, Miller DH, Montalban X, Mowry EM, Sorensen PS, Tintoré M, Traboulsee AL, Trojano M, Uitdehaag BMJ, Vukusic S, Waubant E, Weinshenker BG, Reingold SC, Cohen JA (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 17:162–173. https://doi.org/10.1016/s1474-4422(17)30470-2
Blaschke SJ, Ellenberger D, Flachenecker P, Hellwig K, Paul F, Pöhlau D, Kleinschnitz C, Rommer PS, Rueger MA, Zettl UK, Stahmann A, Warnke C (2022) Time to diagnosis in multiple sclerosis: epidemiological data from the German Multiple Sclerosis Registry. Mult Scler 28:865–871. https://doi.org/10.1177/13524585211039753
Reinhardt K, Weiss S, Rosenbauer J, Gärtner J, von Kries R (2014) Multiple sclerosis in children and adolescents: incidence and clinical picture – new insights from the nationwide German surveillance (2009–2011). Eur J Neurol 21:654–659. https://doi.org/10.1111/ene.12371
Tintore M, Cobo-Calvo A, Carbonell P, Arrambide G, Otero-Romero S, Río J, Tur C, Comabella M, Nos C, Arévalo MJ, Midaglia L, Galán I, Vidal-Jordana A, Castilló J, Rodríguez-Acevedo B, Zabalza de Torres A, Salerno A, Auger C, Sastre-Garriga J, Rovira À, Montalban X (2021) Effect of changes in MS diagnostic criteria over 25 years on time to treatment and prognosis in patients with clinically isolated syndrome. Neurology 97:e1641–e1652. https://doi.org/10.1212/wnl.0000000000012726
Kaisey M, Solomon AJ, Luu M, Giesser BS, Sicotte NL (2019) Incidence of multiple sclerosis misdiagnosis in referrals to two academic centers. Mult Scler Relat Disord 30:51–56. https://doi.org/10.1016/j.msard.2019.01.048
Solomon AJ, Kaisey M, Krieger SC, Chahin S, Naismith RT, Weinstein SM, Shinohara RT, Weinshenker BG (2022) Multiple sclerosis diagnosis: Knowledge gaps and opportunities for educational intervention in neurologists in the United States. Mult Scler 28:1248–1256. https://doi.org/10.1177/13524585211048401
Makhani N, Tremlett H (2021) The multiple sclerosis prodrome. Nat Rev Neurol 17:515–521. https://doi.org/10.1038/s41582-021-00519-3
Bjornevik K, Munger KL, Cortese M, Barro C, Healy BC, Niebuhr DW, Scher AI, Kuhle J, Ascherio A (2020) Serum neurofilament light chain levels in patients with presymptomatic multiple sclerosis. JAMA Neurol 77:58–64. https://doi.org/10.1001/jamaneurol.2019.3238
Yusuf FLA, Ng BC, Wijnands JMA, Kingwell E, Marrie RA, Tremlett H (2020) A systematic review of morbidities suggestive of the multiple sclerosis prodrome. Expert Rev Neurother 20:799–819. https://doi.org/10.1080/14737175.2020.1746645
Marrie RA, Allegretta M, Barcellos LF, Bebo B, Calabresi PA, Correale J, Davis B, De Jager PL, Gasperi C, Greenbaum C, Helme A, Hemmer B, Kanellis P, Kostich W, Landsman D, Lebrun-Frenay C, Makhani N, Munger KL, Okuda DT, Ontaneda D, Postuma RB, Quandt JA, Roman S, Saidha S, Sormani MP, Strum J, Valentine P, Walton C, Zackowski KM, Zhao Y, Tremlett H (2022) From the prodromal stage of multiple sclerosis to disease prevention. Nat Rev Neurol 18:559–572. https://doi.org/10.1038/s41582-022-00686-x
Wijnands JM, Zhu F, Kingwell E, Zhao Y, Ekuma O, Lu X, Evans C, Fisk JD, Marrie RA, Tremlett H (2019) Five years before multiple sclerosis onset: phenotyping the prodrome. Mult Scler 25:1092–1101. https://doi.org/10.1177/1352458518783662
De Stefano N, Giorgio A, Tintore M, Pia Amato M, Kappos L, Palace J, Yousry T, Rocca MA, Ciccarelli O, Enzinger C, Frederiksen J, Filippi M, Vrenken H, Rovira A, group Ms; MAGNIMS study group (2018) Radiologically isolated syndrome or subclinical multiple sclerosis: MAGNIMS consensus recommendations. Mult Scler 24:214–221. https://doi.org/10.1177/1352458517717808
De Stefano N, Stromillo ML, Giorgio A, Bartolozzi ML, Battaglini M, Baldini M, Portaccio E, Amato MP, Sormani MP (2016) Establishing pathological cut-offs of brain atrophy rates in multiple sclerosis. J Neurol Neurosurg Psychiatry 87:93–99. https://doi.org/10.1136/jnnp-2014-309903
Dobson R, Ramagopalan S, Davis A, Giovannoni G (2013) Cerebrospinal fluid oligoclonal bands in multiple sclerosis and clinically isolated syndromes: a meta-analysis of prevalence, prognosis and effect of latitude. J Neurol Neurosurg Psychiatry 84:909–914. https://doi.org/10.1136/jnnp-2012-304695
Gebhardt M, Kropp P, Hoffmann F, Zettl UK (2019) Headache in the course of multiple sclerosis: a prospective study. J Neural Transm (Vienna) 126:131–139. https://doi.org/10.1007/s00702-018-1959-0
Hyncicova E, Kalina A, Vyhnalek M, Nikolai T, Martinkovic L, Lisy J, Hort J, Meluzinova E, Laczó J (2018) Health-related quality of life, neuropsychiatric symptoms and structural brain changes in clinically isolated syndrome. PLoS ONE 13:e0200254. https://doi.org/10.1371/journal.pone.0200254
Hynčicová E, Vyhnálek M, Kalina A, Martinkovič L, Nikolai T, Lisý J, Hort J, Meluzínová E, Laczó J (2017) Cognitive impairment and structural brain changes in patients with clinically isolated syndrome at high risk for multiple sclerosis. J Neurol 264:482–493. https://doi.org/10.1007/s00415-016-8368-9
Kalb R, Beier M, Benedict RH, Charvet L, Costello K, Feinstein A, Gingold J, Goverover Y, Halper J, Harris C, Kostich L, Krupp L, Lathi E, LaRocca N, Thrower B, DeLuca J (2018) Recommendations for cognitive screening and management in multiple sclerosis care. Mult Scler 24:1665–1680. https://doi.org/10.1177/1352458518803785
Lebrun C, Cohen M, Clavelou P, SFSEP (2016) Evaluation of quality of life and fatigue in radiologically isolated syndrome. Rev Neurol (Paris) 172:392–395. https://doi.org/10.1016/j.neurol.2016.04.004
Mañé Martínez MA, Olsson B, Bau L, Matas E, Cobo Calvo Á, Andreasson U, Blennow K, Romero-Pinel L, Martínez-Yélamos S, Zetterberg H (2015) Glial and neuronal markers in cerebrospinal fluid predict progression in multiple sclerosis. Mult Scler 21:550–561. https://doi.org/10.1177/1352458514549397
Matute-Blanch C, Villar LM, Álvarez-Cermeño JC, Rejdak K, Evdoshenko E, Makshakov G, Nazarov V, Lapin S, Midaglia L, Vidal-Jordana A, Drulovic J, Garcia-Merino A, Sanchez-Lopez AJ, Havrdova E, Saiz A, Llufriu S, Alvarez-Lafuente R, Schroeder I, Zettl UK, Galimberti D, Ramio-Torrenta L, Robles R, Quintana E, Hegen H, Deisenhammer F, Rio J, Tintore M, Sanchez A, Montalban X, Comabella M (2018) Neurofilament light chain and oligoclonal bands are prognostic biomarkers in radiologically isolated syndrome. Brain 141:1085–1093. https://doi.org/10.1093/brain/awy021
Mirmosayyeb O, Barzegar M, Nehzat N, Shaygannejad V, Sahraian MA, Ghajarzadeh M (2020) The prevalence of migraine in multiple sclerosis (MS): a systematic review and meta-analysis. J Clin Neurosci 79:33–38. https://doi.org/10.1016/j.jocn.2020.06.021
Momtazmanesh S, Shobeiri P, Saghazadeh A, Teunissen CE, Burman J, Szalardy L, Klivenyi P, Bartos A, Fernandes A, Rezaei N (2021) Neuronal and glial CSF biomarkers in multiple sclerosis: a systematic review and meta-analysis. Rev Neurosci 32:573–595. https://doi.org/10.1515/revneuro-2020-0145
Oliva Ramirez A, Keenan A, Kalau O, Worthington E, Cohen L, Singh S (2021) Prevalence and burden of multiple sclerosis-related fatigue: a systematic literature review. BMC Neurol 21:468. https://doi.org/10.1186/s12883-021-02396-1
Shaygannejad V, Sadeghi Bahmani D, Soleimani P, Mirmosayyeb O, Barzegar M, Amra B, Brand S (2020) Comparison of prevalence rates of restless legs syndrome, self-assessed risks of obstructive sleep apnea, and daytime sleepiness among patients with multiple sclerosis (MS), clinically isolated syndrome (CIS) and neuromyelitis optica spectrum disorder (NMOSD). Sleep Med 70:97–105. https://doi.org/10.1016/j.sleep.2019.11.1266
Vitkova M, Gdovinova Z, Rosenberger J, Szilasiova J, Nagyova I, Mikula P, Krokavcova M, Groothoff JW, van Dijk JP (2014) Factors associated with poor sleep quality in patients with multiple sclerosis differ by disease duration. Disabil Health J 7:466–471. https://doi.org/10.1016/j.dhjo.2014.05.004
Yamout B, Al Khawajah M (2017) Radiologically isolated syndrome and multiple sclerosis. Mult Scler Relat Disord 17:234–237. https://doi.org/10.1016/j.msard.2017.08.016
Cortese M, Riise T, Bjørnevik K, Bhan A, Farbu E, Grytten N, Hogenesch I, Midgard R, Smith Simonsen C, Telstad W, Ascherio A, Myhr KM (2016) Preclinical disease activity in multiple sclerosis: a prospective study of cognitive performance prior to first symptom. Ann Neurol 80:616–624. https://doi.org/10.1002/ana.24769
Lublin FD, Häring DA, Ganjgahi H, Ocampo A, Hatami F, Cuklina J, Aarden P, Dahlke F, Arnold DL, Wiendl H, Chitnis T, Nichols TE, Kieseier BC, Bermel RA (2022) How patients with multiple sclerosis acquire disability. Brain 145:3147–3161. https://doi.org/10.1093/brain/awac016
Portaccio E, Bellinvia A, Fonderico M, Pasto L, Razzolini L, Totaro R, Spitaleri D, Lugaresi A, Cocco E, Onofrj M, Di Palma F, Patti F, Maimone D, Valentino P, Confalonieri P, Protti A, Sola P, Lus G, Maniscalco GT, Brescia Morra V, Salemi G, Granella F, Pesci I, Bergamaschi R, Aguglia U, Vianello M, Simone M, Lepore V, Iaffaldano P, Filippi M, Trojano M, Amato MP (2022) Progression is independent of relapse activity in early multiple sclerosis: a real-life cohort study. Brain 145:2796–2805. https://doi.org/10.1093/brain/awac111
Gärtner J, Hauser SL, Bar-Or A, Montalban X, Cohen JA, Cross AH, Deiva K, Ganjgahi H, Haring DA, Li B, Pingili R, Ramanathan K, Su W, Willi R, Kieseier B, Kappos L (2022) Efficacy and safety of ofatumumab in recently diagnosed, treatment-naive patients with multiple sclerosis: results from ASCLEPIOS I and II. Mult Scler 28:1562–1575. https://doi.org/10.1177/13524585221078825
Marin CE, Kfouri PP, Callegaro D, Lana-Peixoto MA, Neto APG, Vasconcelos CCF, d’Almeida JAC, Goncalves MVM, Mendes MF, Parolin MKF, Nascimento O, da Gama PD, Dias-Carneiro RPC, Dias RM, Damasceno A, San Martin G, Becker J, Brazilian Committee for T, Research in Multiple S (2021) Patients and neurologists have different perceptions of multiple sclerosis symptoms, care and challenges. Mult Scler Relat Disord 50:102806. https://doi.org/10.1016/j.msard.2021.102806
Zipoli V, Goretti B, Hakiki B, Siracusa G, Sorbi S, Portaccio E, Amato MP (2010) Cognitive impairment predicts conversion to multiple sclerosis in clinically isolated syndromes. Mult Scler 16:62–67. https://doi.org/10.1177/1352458509350311
Schulz D, Kopp B, Kunkel A, Faiss JH (2006) Cognition in the early stage of multiple sclerosis. J Neurol 253:1002–1010. https://doi.org/10.1007/s00415-006-0145-8
Migliore S, Ghazaryan A, Simonelli I, Pasqualetti P, Squitieri F, Curcio G, Landi D, Palmieri MG, Moffa F, Filippi MM, Vernieri F (2017) Cognitive impairment in relapsing-remitting multiple sclerosis patients with very mild clinical disability. Behav Neurol 2017:7404289. https://doi.org/10.1155/2017/7404289
Brissart H, Morele E, Baumann C, Perf ML, Leininger M, Taillemite L, Dillier C, Pittion S, Spitz E, Debouverie M (2013) Cognitive impairment among different clinical courses of multiple sclerosis. Neurol Res 35:867–872. https://doi.org/10.1179/1743132813Y.0000000232
Sinay V, Perez Akly M, Zanga G, Ciardi C, Racosta JM (2015) School performance as a marker of cognitive decline prior to diagnosis of multiple sclerosis. Mult Scler 21:945–952. https://doi.org/10.1177/1352458514554054
Simonsen CS, Flemmen HØ, Broch L, Brunborg C, Berg-Hansen P, Moen SM, Celius EG (2021) No significant differences in absenteeism or academic achievements in a Norwegian multiple sclerosis case control study. Mult Scler Relat Disord 54:103141. https://doi.org/10.1016/j.msard.2021.103141
Uher T, Krasensky J, Sobisek L, Blahova Dusankova J, Seidl Z, Kubala Havrdova E, Sormani MP, Horakova D, Kalincik T, Vaneckova M (2018) Cognitive clinico-radiological paradox in early stages of multiple sclerosis. Ann Clin Transl Neurol 5:81–91. https://doi.org/10.1002/acn3.512
Pitteri M, Magliozzi R, Nicholas R, Ziccardi S, Pisani AI, Pezzini F, Marastoni D, Calabrese M (2022) Cerebrospinal fluid inflammatory profile of cognitive impairment in newly diagnosed multiple sclerosis patients. Mult Scler 28:768–777. https://doi.org/10.1177/13524585211032510
Rocca MA, Amato MP, De Stefano N, Enzinger C, Geurts JJ, Penner IK, Rovira A, Sumowski JF, Valsasina P, Filippi M, MAGNIMS Study Group (2015) Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol 14:302–317. https://doi.org/10.1016/S1474-4422(14)70250-9
López-Góngora M, Escartín A, Martínez-Horta S, Fernández-Bobadilla R, Querol L, Romero S, Mañanas M, Riba J (2015) Neurophysiological Evidence of Compensatory Brain Mechanisms in Early-Stage Multiple Sclerosis. PLoS ONE 10:e0136786. https://doi.org/10.1371/journal.pone.0136786
Oset M, Stasiolek M, Matysiak M (2020) Cognitive dysfunction in the early stages of multiple sclerosis-how much and how important? Curr Neurol Neurosci Rep 20:22. https://doi.org/10.1007/s11910-020-01045-3
Meca-Lallana V, Gascón-Giménez F, Ginestal-López RC, Higueras Y, Tellez-Lara N, Carreres-Polo J, Eichau-Madueno S, Romero-Imbroda J, Vidal-Jordana A, Perez-Miralles F (2021) Cognitive impairment in multiple sclerosis: diagnosis and monitoring. Neurol Sci 42:5183–5193. https://doi.org/10.1007/s10072-021-05165-7
Corfield F, Langdon D (2018) A systematic review and meta-analysis of the brief cognitive assessment for multiple sclerosis (BICAMS). Neurol Ther 7:287–306. https://doi.org/10.1007/s40120-018-0102-3
Rao SM, Losinski G, Mourany L, Schindler D, Mamone B, Reece C, Kemeny D, Narayanan S, Miller DM, Bethoux F, Bermel RA, Rudick R, Alberts J (2017) Processing speed test: validation of a self-administered, iPad®-based tool for screening cognitive dysfunction in a clinic setting. Mult Scler 23:1929–1937. https://doi.org/10.1177/1352458516688955
Benedict RH (2005) Effects of using same- versus alternate-form memory tests during short-interval repeated assessments in multiple sclerosis. J Int Neuropsychol Soc 11:727–736. https://doi.org/10.1017/S1355617705050782
Yusuf F, Wijnands JM, Kingwell E, Zhu F, Evans C, Fisk JD, Zhao Y, Sutherland JM, Patrick DM, Marrie RA, Tremlett H (2021) Fatigue, sleep disorders, anaemia and pain in the multiple sclerosis prodrome. Mult Scler 27:290–302. https://doi.org/10.1177/1352458520908163
Marrie RA, Reingold S, Cohen J, Stuve O, Trojano M, Sorensen PS, Cutter G, Reider N (2015) The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler 21:305–317. https://doi.org/10.1177/1352458514564487
Heesen C, Schulz KH, Fiehler J, Von der Mark U, Otte C, Jung R, Poettgen J, Krieger T, Gold SM (2010) Correlates of cognitive dysfunction in multiple sclerosis. Brain Behav Immun 24:1148–1155. https://doi.org/10.1016/j.bbi.2010.05.006
Manjaly Z-M, Harrison NA, Critchley HD, Do CT, Stefanics G, Wenderoth N, Lutterotti A, Muller A, Stephan KE (2019) Pathophysiological and cognitive mechanisms of fatigue in multiple sclerosis. J Neurol Neurosurg Psychiatry 90:642–651. https://doi.org/10.1136/jnnp-2018-320050
Murphy R, O’Donoghue S, Counihan T, McDonald C, Calabresi PA, Ahmed MA, Kaplin A, Hallahan B (2017) Neuropsychiatric syndromes of multiple sclerosis. J Neurol Neurosurg Psychiatry 88:697–708. https://doi.org/10.1136/jnnp-2016-315367
Carmosino MJ, Brousseau KM, Arciniegas DB, Corboy JR (2005) Initial evaluations for multiple sclerosis in a university multiple sclerosis center: outcomes and role of magnetic resonance imaging in referral. Arch Neurol 62:585–590. https://doi.org/10.1001/archneur.62.4.585
Solomon AJ, Naismith RT, Cross AH (2019) Misdiagnosis of multiple sclerosis: impact of the 2017 McDonald criteria on clinical practice. Neurology 92:26–33. https://doi.org/10.1212/WNL.0000000000006583
Kelly SB, Chaila E, Kinsella K, Duggan M, Walsh C, Tubridy N, Hutchinson M (2012) Using atypical symptoms and red flags to identify non-demyelinating disease. J Neurol Neurosurg Psychiatry 83:44–48. https://doi.org/10.1136/jnnp-2011-300679
Beswick E, Quigley S, Macdonald P, Patrick S, Colville S, Chandran S, Connick P (2022) The Patient Health Questionnaire (PHQ-9) as a tool to screen for depression in people with multiple sclerosis: a cross-sectional validation study. BMC Psychol 10:281. https://doi.org/10.1186/s40359-022-00949-8
Karrenbauer VD, Bedri SK, Hillert J, Manouchehrinia A (2021) Cerebrospinal fluid oligoclonal immunoglobulin gamma bands and long-term disability progression in multiple sclerosis: a retrospective cohort study. Sci Rep 11:14987. https://doi.org/10.1038/s41598-021-94423-x
Perrone C, Berger JR, Markowotz C (2018) Oligoclonal band number correlates with relapses and progression in multiple sclerosis. Presented at the Consortium of Multiple Sclerosis Centers (CMSC) Annual Meeting, May 30-June 2, Nashville, TN, USA
Ziemssen T, Akgun K, Bruck W (2019) Molecular biomarkers in multiple sclerosis. J Neuroinflammation 16:272. https://doi.org/10.1186/s12974-019-1674-2
Bonnan M (2015) Intrathecal IgG synthesis: a resistant and valuable target for future multiple sclerosis treatments. Mult Scler Int 2015:296184. https://doi.org/10.1155/2015/296184
Cai L, Huang J (2018) Neurofilament light chain as a biological marker for multiple sclerosis: a meta-analysis study. Neuropsychiatr Dis Treat 14:2241–2254. https://doi.org/10.2147/ndt.S173280
Kouchaki E, Dashti F, Mirazimi SMA, Alirezaei Z, Jafari SH, Hamblin MR, Mirzaei H (2021) Neurofilament light chain as a biomarker for diagnosis of multiple sclerosis. EXCLI J 20:1308–1325. https://doi.org/10.17179/excli2021-3973
Yuan A, Sasaki T, Kumar A, Peterhoff CM, Rao MV, Liem RK, Julien JP, Nixon RA (2012) Peripherin is a subunit of peripheral nerve neurofilaments: implications for differential vulnerability of CNS and peripheral nervous system axons. J Neurosci 32:8501–8508. https://doi.org/10.1523/JNEUROSCI.1081-12.2012
Teunissen CE, Khalil M (2012) Neurofilaments as biomarkers in multiple sclerosis. Mult Scler 18:552–556. https://doi.org/10.1177/1352458512443092
Chitnis T, Gonzalez C, Healy BC, Saxena S, Rosso M, Barro C, Michalak Z, Paul A, Kivisakk P, Diaz-Cruz C, Sattarnezhad N, Pierre IV, Glanz BI, Tomic D, Kropshofer H, Haring D, Leppert D, Kappos L, Bakshi R, Weiner HL, Kuhle J (2018) Neurofilament light chain serum levels correlate with 10-year MRI outcomes in multiple sclerosis. Ann Clin Transl Neurol 5:1478–1491. https://doi.org/10.1002/acn3.638
Salzer J, Svenningsson A, Sundstrom P (2010) Neurofilament light as a prognostic marker in multiple sclerosis. Mult Scler 16:287–292. https://doi.org/10.1177/1352458509359725
Gaetani L, Eusebi P, Mancini A, Gentili L, Borrelli A, Parnetti L, Calabresi P, Sarchielli P, Blennow K, Zetterberg H, Di Filippo M (2019) Cerebrospinal fluid neurofilament light chain predicts disease activity after the first demyelinating event suggestive of multiple sclerosis. Mult Scler Relat Disord 35:228–232. https://doi.org/10.1016/j.msard.2019.07.025
Bhan A, Jacobsen C, Dalen I, Bergsland N, Zivadinov R, Alves G, Myhr KM, Farbu E (2021) CSF neurofilament light chain predicts 10-year clinical and radiologic worsening in multiple sclerosis. Mult Scler J Exp Transl Clin 7:20552173211060336. https://doi.org/10.1177/20552173211060337
Srpova B, Uher T, Hrnciarova T, Barro C, Andelova M, Michalak Z, Vaneckova M, Krasensky J, Noskova L, Havrdova EK, Kuhle J, Horakova D (2021) Serum neurofilament light chain reflects inflammation-driven neurodegeneration and predicts delayed brain volume loss in early stage of multiple sclerosis. Mult Scler 27:52–60. https://doi.org/10.1177/1352458519901272
Benkert P, Meier S, Schaedelin S, Manouchehrinia A, Yaldizli O, Maceski A, Oechtering J, Achtnichts L, Conen D, Derfuss T, Lalive PH, Mueller C, Muller S, Naegelin Y, Oksenberg JR, Pot C, Salmen A, Willemse E, Kockum I, Blennow K, Zetterberg H, Gobbi C, Kappos L, Wiendl H, Berger K, Sormani MP, Granziera C, Piehl F, Leppert D, Kuhle J, Nf LRDitSMSCSG, (2022) Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: a retrospective modelling and validation study. Lancet Neurol 21:246–257. https://doi.org/10.1016/S1474-4422(22)00009-6
Ziemssen T, Arnold DL, Alvarez E, Cross AH, Willi R, Li B, Kukkaro P, Kropshofer H, Ramanathan K, Merschhemke M, Kieseier B, Su W, Haring DA, Hauser SL, Kappos L, Kuhle J (2022) Prognostic value of serum neurofilament light chain for disease activity and worsening in patients with relapsing multiple sclerosis: results from the phase 3 ASCLEPIOS I and II trials. Front Immunol 13:852563. https://doi.org/10.3389/fimmu.2022.852563
Freedman MS, Gnanapavan S (2021) CMSC consensus statement on neurofilament biomarkers in multiple sclerosis. Int J MS Care 23(suppl 1):2–32
Salzer J, Svenningsson A, Sundström P (2010) Neurofilament light as a prognostic marker in multiple sclerosis. Mult Scler 16:287–292. https://doi.org/10.1177/1352458509359725
Sen MK, Hossain MJ, Mahns DA, Brew BJ (2022) Validity of serum neurofilament light chain as a prognostic biomarker of disease activity in multiple sclerosis. J Neurol. https://doi.org/10.1007/s00415-022-11507-y
Akamine S, Marutani N, Kanayama D, Gotoh S, Maruyama R, Yanagida K, Sakagami Y, Mori K, Adachi H, Kozawa J, Maeda N, Otsuki M, Matsuoka T, Iwahashi H, Shimomura I, Ikeda M, Kudo T (2020) Renal function is associated with blood neurofilament light chain level in older adults. Sci Rep 10:20350. https://doi.org/10.1038/s41598-020-76990-7
Saraste M, Bezukladova S, Matilainen M, Sucksdorff M, Kuhle J, Leppert D, Airas L (2021) Increased serum glial fibrillary acidic protein associates with microstructural white matter damage in multiple sclerosis: GFAP and DTI. Mult Scler Relat Disord 50:102810. https://doi.org/10.1016/j.msard.2021.102810
Karimi N, Ashourizadeh H, Akbarzadeh Pasha B, Haghshomar M, Jouzdani T, Shobeiri P, Teixeira AL, Rezaei N (2022) Blood levels of brain-derived neurotrophic factor (BDNF) in people with multiple sclerosis (MS): a systematic review and meta-analysis. Mult Scler Relat Disord 65:103984. https://doi.org/10.1016/j.msard.2022.103984
Yalachkov Y, Anschütz V, Jakob J, Schaller-Paule MA, Schäfer JH, Reiländer A, Friedauer L, Behrens M, Steffen F, Bittner S, Foerch C (2022) Brain-derived neurotrophic factor and neurofilament light chain in cerebrospinal fluid are inversely correlated with cognition in multiple sclerosis at the time of diagnosis. Mult Scler Relat Disord 63:103822. https://doi.org/10.1016/j.msard.2022.103822
Vollmer T, Signorovitch J, Huynh L, Galebach P, Kelley C, DiBernardo A, Sasane R (2015) The natural history of brain volume loss among patients with multiple sclerosis: a systematic literature review and meta-analysis. J Neurol Sci 357:8–18. https://doi.org/10.1016/j.jns.2015.07.014
De Stefano N, Airas L, Grigoriadis N, Mattle HP, O’Riordan J, Oreja-Guevara C, Sellebjerg F, Stankoff B, Walczak A, Wiendl H, Kieseier BC (2014) Clinical relevance of brain volume measures in multiple sclerosis. CNS Drugs 28:147–156. https://doi.org/10.1007/s40263-014-0140-z
Andorra M, Nakamura K, Lampert EJ, Pulido-Valdeolivas I, Zubizarreta I, Llufriu S, Martinez-Heras E, Sola-Valls N, Sepulveda M, Tercero-Uribe A, Blanco Y, Saiz A, Villoslada P, Martinez-Lapiscina EH (2018) Assessing biological and methodological aspects of brain volume loss in multiple sclerosis. JAMA Neurol 75:1246–1255. https://doi.org/10.1001/jamaneurol.2018.1596
Chard DT, Brex PA, Ciccarelli O, Griffin CM, Parker GJ, Dalton C, Altmann DR, Thompson AJ, Miller DH (2003) The longitudinal relation between brain lesion load and atrophy in multiple sclerosis: a 14 year follow up study. J Neurol Neurosurg Psychiatry 74:1551–1554. https://doi.org/10.1136/jnnp.74.11.1551
Ziccardi S, Pizzini FB, Guandalini M, Tamanti A, Cristofori C, Calabrese M (2022) Making visible the invisible: automatically measured global and regional brain volume is associated with cognitive impairment and fatigue in multiple sclerosis. Bioengineering (Basel). https://doi.org/10.3390/bioengineering10010041
Zivadinov R, Jakimovski D, Gandhi S, Ahmed R, Dwyer MG, Horakova D, Weinstock-Guttman B, Benedict RR, Vaneckova M, Barnett M, Bergsland N (2016) Clinical relevance of brain atrophy assessment in multiple sclerosis. Implications for its use in a clinical routine. Expert Rev Neurother 16:777–793. https://doi.org/10.1080/14737175.2016.1181543
Absinta M, Sati P, Masuzzo F, Nair G, Sethi V, Kolb H, Ohayon J, Wu T, Cortese ICM, Reich DS (2019) Association of chronic active multiple sclerosis lesions with disability in vivo. JAMA Neurol 76:1474–1483. https://doi.org/10.1001/jamaneurol.2019.2399
Wittayer M, Weber CE, Kittel M, Platten M, Schirmer L, Tumani H, Gass A, Eisele P (2023) Cerebrospinal fluid-related tissue damage in multiple sclerosis patients with iron rim lesions. Mult Scler 29:549–558. https://doi.org/10.1177/13524585231155639
Ng Kee Kwong KC, Mollison D, Meijboom R, York EN, Kampaite A, Martin SJ, Hunt DPJ, Thrippleton MJ, Chandran S, Waldman AD, Future MSc (2022) Rim lesions are demonstrated in early relapsing-remitting multiple sclerosis using 3 T-based susceptibility-weighted imaging in a multi-institutional setting. Neuroradiology 64:109–117. https://doi.org/10.1007/s00234-021-02768-x
Absinta M, Maric D, Gharagozloo M, Garton T, Smith MD, Jin J, Fitzgerald KC, Song A, Liu P, Lin JP, Wu T, Johnson KR, McGavern DB, Schafer DP, Calabresi PA, Reich DS (2021) A lymphocyte-microglia-astrocyte axis in chronic active multiple sclerosis. Nature 597:709–714. https://doi.org/10.1038/s41586-021-03892-7
Calvi A, Clarke MA, Prados F, Chard D, Ciccarelli O, Alberich M, Pareto D, Rodriguez Barranco M, Sastre-Garriga J, Tur C, Rovira A, Barkhof F (2023) Relationship between paramagnetic rim lesions and slowly expanding lesions in multiple sclerosis. Mult Scler 29:352–362. https://doi.org/10.1177/13524585221141964
Elliott C, Wolinsky JS, Hauser SL, Kappos L, Barkhof F, Bernasconi C, Wei W, Belachew S, Arnold DL (2019) Slowly expanding/evolving lesions as a magnetic resonance imaging marker of chronic active multiple sclerosis lesions. Mult Scler 25:1915–1925. https://doi.org/10.1177/1352458518814117
Preziosa P, Pagani E, Meani A, Moiola L, Rodegher M, Filippi M, Rocca MA (2022) Slowly Expanding lesions predict 9-year multiple sclerosis disease progression. Neurol Neuroimmunol Neuroinflamm. https://doi.org/10.1212/NXI.0000000000001139
Elliott C, Rudko DA, Arnold DL, Fetco D, Elkady AM, Araujo D, Zhu B, Gafson A, Tian Z, Belachew S, Bradley DP, Fisher E (2023) Lesion-level correspondence and longitudinal properties of paramagnetic rim and slowly expanding lesions in multiple sclerosis. Mult Scler 29:680–690. https://doi.org/10.1177/13524585231162262
Martire MS, Moiola L, Rocca MA, Filippi M, Absinta M (2022) What is the potential of paramagnetic rim lesions as diagnostic indicators in multiple sclerosis? Expert Rev Neurother 22:829–837. https://doi.org/10.1080/14737175.2022.2143265
Maggi P, Absinta M, Sati P, Perrotta G, Massacesi L, Dachy B, Pot C, Meuli R, Reich DS, Filippi M, Pasquier RD, Theaudin M (2020) The “central vein sign” in patients with diagnostic “red flags” for multiple sclerosis: a prospective multicenter 3T study. Mult Scler 26:421–432. https://doi.org/10.1177/1352458519876031
Clarke MA, Pareto D, Pessini-Ferreira L, Arrambide G, Alberich M, Crescenzo F, Cappelle S, Tintore M, Sastre-Garriga J, Auger C, Montalban X, Evangelou N, Rovira A (2020) Value of 3T susceptibility-weighted imaging in the diagnosis of multiple sclerosis. AJNR Am J Neuroradiol 41:1001–1008. https://doi.org/10.3174/ajnr.A6547
Maggi P, Sati P, Nair G, Cortese ICM, Jacobson S, Smith BR, Nath A, Ohayon J, van Pesch V, Perrotta G, Pot C, Theaudin M, Martinelli V, Scotti R, Wu T, Du Pasquier R, Calabresi PA, Filippi M, Reich DS, Absinta M (2020) Paramagnetic rim lesions are specific to multiple sclerosis: an international multicenter 3T MRI study. Ann Neurol 88:1034–1042. https://doi.org/10.1002/ana.25877
Wiendl H, Gold R, Zipp F, Multiple Sclerosis Therapy Consensus Group (2021) Multiple Sclerosis Therapy Consensus Group (MSTCG): answers to the discussion questions. Neurol Res Pract 3:44. https://doi.org/10.1186/s42466-021-00140-1
Giovannoni G (2019) Do we have equipoise when it comes to how we treat active multiple sclerosis? Lancet Neurol 18:909–911. https://doi.org/10.1016/S1474-4422(19)30227-3
Okuda DT, Kantarci O, Lebrun-Frénay C, Sormani MP, Azevedo CJ, Bovis F, Hua LH, Amezcua L, Mowry EM, Hotermans C, Mendoza J, Walsh JS, von Hehn C, Vargas WS, Donlon S, Naismith RT, Okai A, Pardo G, Repovic P, Stüve O, Siva A, Pelletier D (2022) Multi-center, randomized, double-blinded assessment of dimethyl fumarate in extending the time to a first clinical demyelinating event in radiologically isolated syndrome (ARISE). Mult Scler 28(suppl 3):950–951. https://doi.org/10.1177/13524585221126908
Cobo-Calvo A, Tur C, Otero-Romero S, Carbonell-Mirabent P, Ruiz M, Pappolla A, Alvarez JV, Vidal-Jordana A, Arrambide G, Castillo J, Galan I, Rodriguez Barranco M, Midaglia LS, Nos C, Rodriguez Acevedo B, Zabalza de Torres A, Mongay N, Rio J, Comabella M, Auger C, Sastre-Garriga J, Rovira A, Tintore M, Montalban X (2023) Association of very early treatment initiation with the risk of long-term disability in patients with a first demyelinating event. Neurology. https://doi.org/10.1212/WNL.0000000000207664
ClinicalTrials.gov (2022) Randomized, Double-blinded Study of Treatment:Teriflunomide, in Radiologically Isolated Syndrome (TERIS) [NCT03122652]. https://clinicaltrials.gov/ct2/show/NCT03122652?cond=Radiologically+Isolated+Syndrome&draw=2&rank=5. Accessed October 31 2022
Longbrake EE, Hua LH, Mowry EM, Gauthier SA, Alvarez E, Cross AH, Pei J, Priest J, Raposo C, Hafler DA, Winger RC (2022) The CELLO trial: protocol of a planned phase 4 study to assess the efficacy of ocrelizumab in patients with radiologically isolated syndrome. Mult Scler Relat Disord 68:104143. https://doi.org/10.1016/j.msard.2022.104143
Filippi M, Danesi R, Derfuss T, Duddy M, Gallo P, Gold R, Havrdová EK, Kornek B, Saccà F, Tintoré M, Weber J, Trojano M (2022) Early and unrestricted access to high-efficacy disease-modifying therapies: a consensus to optimize benefits for people living with multiple sclerosis. J Neurol 269:1670–1677. https://doi.org/10.1007/s00415-021-10836-8
TG Therapeutics. Briumvi (ublituximab) [package insert]. U.S. Food and Drug Administration website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761238s000lbl.pdf Revised December 2022. Accessed June 30, 2023.
Freeman L, Longbrake EE, Coyle PK, Hendin B, Vollmer T (2022) High-efficacy therapies for treatment-naive individuals with relapsing-remitting multiple sclerosis. CNS Drugs 36:1285–1299. https://doi.org/10.1007/s40263-022-00965-7
Labiano-Fontcuberta A, Costa-Frossard L, Sainz de la Maza S, Rodríguez-Jorge F, Chico-García JL, Monreal E (2022) The effect of timing of high-efficacy therapy on processing speed performance in multiple sclerosis. Mult Scler Relat Disord 64:103959. https://doi.org/10.1016/j.msard.2022.103959
He A, Merkel B, Brown JWL, Zhovits Ryerson L, Kister I, Malpas CB, Sharmin S, Horakova D, Kubala Havrdova E, Spelman T, Izquierdo G, Eichau S, Trojano M, Lugaresi A, Hupperts R, Sola P, Ferraro D, Lycke J, Grand’Maison F, Prat A, Girard M, Duquette P, Larochelle C, Svenningsson A, Petersen T, Grammond P, Granella F, Van Pesch V, Bergamaschi R, McGuigan C, Coles A, Hillert J, Piehl F, Butzkueven H, Kalincik T (2020) Timing of high-efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol 19:307–316. https://doi.org/10.1016/s1474-4422(20)30067-3
Harding K, Williams O, Willis M, Hrastelj J, Rimmer A, Joseph F, Tomassini V, Wardle M, Pickersgill T, Robertson N, Tallantyre E (2019) Clinical outcomes of escalation vs early intensive disease-modifying therapy in patients with multiple sclerosis. JAMA Neurol 76:536–541. https://doi.org/10.1001/jamaneurol.2018.4905
Buron MD, Chalmer TA, Sellebjerg F, Barzinji I, Christensen JR, Christensen MK, Hansen V, Illes Z, Jensen HB, Kant M, Papp V, Petersen T, Rasmussen PV, Schäfer J, Theódórsdóttir Á, Weglewski A, Sorensen PS, Magyari M (2020) Initial high-efficacy disease-modifying therapy in multiple sclerosis: a nationwide cohort study. Neurology 95:e1041–e1051. https://doi.org/10.1212/wnl.0000000000010135
Brown JWL, Coles A, Horakova D, Havrdova E, Izquierdo G, Prat A, Girard M, Duquette P, Trojano M, Lugaresi A, Bergamaschi R, Grammond P, Alroughani R, Hupperts R, McCombe P, Van Pesch V, Sola P, Ferraro D, Grand’Maison F, Terzi M, Lechner-Scott J, Flechter S, Slee M, Shaygannejad V, Pucci E, Granella F, Jokubaitis V, Willis M, Rice C, Scolding N, Wilkins A, Pearson OR, Ziemssen T, Hutchinson M, Harding K, Jones J, McGuigan C, Butzkueven H, Kalincik T, Robertson N, MSBase Study Group (2019) Association of initial disease-modifying therapy with later conversion to secondary progressive multiple sclerosis. JAMA 321:175–187. https://doi.org/10.1001/jama.2018.20588
Iaffaldano P, Lucisano G, Caputo F, Paolicelli D, Patti F, Zaffaroni M, Brescia Morra V, Pozzilli C, De Luca G, Inglese M, Salemi G, Maniscalco GT, Cocco E, Sola P, Lus G, Conte A, Amato MP, Granella F, Gasperini C, Bellantonio P, Totaro R, Rovaris M, Salvetti M, Torri Clerici VLA, Bergamaschi R, Maimone D, Scarpini E, Capobianco M, Comi G, Filippi M, Trojano M (2021) Long-term disability trajectories in relapsing multiple sclerosis patients treated with early intensive or escalation treatment strategies. Ther Adv Neurol Disord 14:17562864211019574. https://doi.org/10.1177/17562864211019574
Rojas JI, Patrucco L, Alonso R, Garcea O, Deri N, Carnero Contentti E, Lopez PA, Pettinicchi JP, Caride A, Cristiano E (2022) Effectiveness and safety of early high-efficacy versus escalation therapy in relapsing-remitting multiple sclerosis in Argentina. Clin Neuropharmacol 45:45–51. https://doi.org/10.1097/wnf.0000000000000503
Merkel B, Butzkueven H, Traboulsee AL, Havrdova E, Kalincik T (2017) Timing of high-efficacy therapy in relapsing-remitting multiple sclerosis: a systematic review. Autoimmun Rev 16:658–665. https://doi.org/10.1016/j.autrev.2017.04.010
Sorensen PS, Fox RJ, Comi G (2020) The window of opportunity for treatment of progressive multiple sclerosis. Curr Opin Neurol 33:262–270. https://doi.org/10.1097/wco.0000000000000811
Weideman AM, Tapia-Maltos MA, Johnson K, Greenwood M, Bielekova B (2017) Meta-analysis of the age-dependent efficacy of multiple sclerosis treatments. Front Neurol 8:577. https://doi.org/10.3389/fneur.2017.00577
Kappos L, Wolinsky JS, Giovannoni G, Arnold DL, Wang Q, Bernasconi C, Model F, Koendgen H, Manfrini M, Belachew S, Hauser SL (2020) Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol 77:1132–1140. https://doi.org/10.1001/jamaneurol.2020.1568
Cree BAC, Hollenbach JA, Bove R, Kirkish G, Sacco S, Caverzasi E, Bischof A, Gundel T, Zhu AH, Papinutto N, Stern WA, Bevan C, Romeo A, Goodin DS, Gelfand JM, Graves J, Green AJ, Wilson MR, Zamvil SS, Zhao C, Gomez R, Ragan NR, Rush GQ, Barba P, Santaniello A, Baranzini SE, Oksenberg JR, Henry RG, Hauser SL, University of California SFM-ET (2019) Silent progression in disease activity-free relapsing multiple sclerosis. Ann Neurol 85:653–666. https://doi.org/10.1002/ana.25463
Ontaneda D, Tallantyre EC, Raza PC, Planchon SM, Nakamura K, Miller D, Hersh C, Craner M, Bale C, Chaudhry B, Gunzler DD, Love TE, Gerry S, Coles A, Cohen JA, Evangelou N (2020) Determining the effectiveness of early intensive versus escalation approaches for the treatment of relapsing-remitting multiple sclerosis: The DELIVER-MS study protocol. Contemp Clin Trials 95:106009. https://doi.org/10.1016/j.cct.2020.106009
ClinicalTrials.gov (2022) Traditional Versus Early Aggressive Therapy for Multiple Sclerosis Trial (TREAT-MS) [NCT03500328]. https://clinicaltrials.gov/ct2/show/NCT03500328?term=NCT03500328&draw=2&rank=1. Accessed October 28 2022
Clinicaltrials.gov (2022) Determining the Effectiveness of earLy Intensive Versus Escalation Approaches for RRMS (DELIVER-MS). https://clinicaltrials.gov/ct2/show/NCT03535298. Accessed March 14 2023
Langer-Gould A, Klocke S, Beaber B, Brara SM, Debacker J, Ayeni O, Nielsen AS (2021) Improving quality, affordability, and equity of multiple sclerosis care. Ann Clin Transl Neurol 8:980–991. https://doi.org/10.1002/acn3.51326
Bourdette DN, Hartung DM, Whitham RH (2016) Practices of US health insurance companies concerning MS therapies interfere with shared decision-making and harm patients. Neurol Clin Pract 6:177–182. https://doi.org/10.1212/CPJ.0000000000000208
Filippi M, Amato MP, Centonze D, Gallo P, Gasperini C, Inglese M, Patti F, Pozzilli C, Preziosa P, Trojano M (2022) Early use of high-efficacy disease-modifying therapies makes the difference in people with multiple sclerosis: an expert opinion. J Neurol 269:5382–5394. https://doi.org/10.1007/s00415-022-11193-w
Heesen C, Kleiter I, Meuth SG, Krämer J, Kasper J, Köpke S, Gaissmaier W (2017) Benefit-risk perception of natalizumab therapy in neurologists and a large cohort of multiple sclerosis patients. J Neurol Sci 376:181–190. https://doi.org/10.1016/j.jns.2017.03.001
Calabrese M, Marastoni D, Crescenzo F, Scalfari A (2021) Early multiple sclerosis: diagnostic challenges in clinically and radiologically isolated syndrome patients. Curr Opin Neurol 34:277–285. https://doi.org/10.1097/WCO.0000000000000921
Glasmacher SA, Kearns PK, Hassan Z, Connick P, Tauber S, Reetz K, Foley P, Chandran S, Future MSC (2022) The influence of disease-modifying therapy on hidden disability burden in people with newly diagnosed relapsing-remitting multiple sclerosis. Mult Scler Relat Disord 63:103837. https://doi.org/10.1016/j.msard.2022.103837
Rieckmann P, Centonze D, Elovaara I, Giovannoni G, Havrdova E, Kesselring J, Kobelt G, Langdon D, Morrow SA, Oreja-Guevara C, Schippling S, Thalheim C, Thompson H, Vermersch P, Aston K, Bauer B, Demory C, Giambastiani MP, Hlavacova J, Nouvet-Gire J, Pepper G, Pontaga M, Rogan E, Rogalski C, van Galen P, Ben-Amor AF, Members of the MS in the 21st Century Steering Group (2018) Unmet needs, burden of treatment, and patient engagement in multiple sclerosis: a combined perspective from the MS in the 21st Century Steering Group. Mult Scler Relat Disord 19:153–160. https://doi.org/10.1016/j.msard.2017.11.013
Giovannoni G, Turner B, Gnanapavan S, Offiah C, Schmierer K, Marta M (2015) Is it time to target no evident disease activity (NEDA) in multiple sclerosis? Mult Scler Relat Disord 4:329–333. https://doi.org/10.1016/j.msard.2015.04.006
Damasceno A, Damasceno BP, Cendes F (2016) No evidence of disease activity in multiple sclerosis: implications on cognition and brain atrophy. Mult Scler 22:64–72. https://doi.org/10.1177/1352458515604383
Cree BA, Gourraud P-A, Oksenberg JR, Bevan C, Crabtree-Hartman E, Gelfand JM, Goodin DS, Graves J, Green AJ, Mowry E, Okuda DT, Pelletier D, von Budingen HC, Zamvil SS, Agrawal A, Caillier S, Ciocca C, Gomez R, Kanner R, Lincoln R, Lizee A, Qualley P, Santaniello A, Suleiman L, Bucci M, Panara V, Papinutto N, Stern WA, Zhu AH, Cutter GR, Baranzini S, Henry RG, Hauser SL (2016) Long-term evolution of multiple sclerosis disability in the treatment era. Ann Neurol 80:499–510. https://doi.org/10.1002/ana.24747
Kappos L, De Stefano N, Freedman MS, Cree BA, Radue EW, Sprenger T, Sormani MP, Smith T, Haring DA, Piani Meier D, Tomic D (2016) Inclusion of brain volume loss in a revised measure of “no evidence of disease activity” (NEDA-4) in relapsing-remitting multiple sclerosis. Mult Scler 22:1297–1305. https://doi.org/10.1177/1352458515616701
Szilasiová J, Rosenberger J, Fedičová M, Mikula P, Urban P, Gdovinova Z, Vitkova M, Hanes J, Stevens E (2021) Neurofilament light chain levels are associated with disease activity determined by no evident disease activity in multiple sclerosis patients. Eur Neurol 84:272–279. https://doi.org/10.1159/000515806
Hakansson I, Tisell A, Cassel P, Blennow K, Zetterberg H, Lundberg P, Dahle C, Vrethem M, Ernerudh J (2018) Neurofilament levels, disease activity and brain volume during follow-up in multiple sclerosis. J Neuroinflammation 15:209. https://doi.org/10.1186/s12974-018-1249-7
Guevara C, Villa E, Diaz V, Garrido C, Martinez M, Orellana P, Alarcon P, Silva-Rosas C, Barker GJ, Kempton MJ, de Grazia J (2020) Inclusion of the Symbol Digit Modalities Test in a revised assessment of “no evidence of disease activity-4 (NEDA-4)” in Latin-American patients with multiple sclerosis. Mult Scler Relat Disord 42:102076. https://doi.org/10.1016/j.msard.2020.102076
Kappos L, Fox RJ, Burcklen M, Freedman MS, Havrdova EK, Hennessy B, Hohlfeld R, Lublin F, Montalban X, Pozzilli C, Scherz T, D’Ambrosio D, Linscheid P, Vaclavkova A, Pirozek-Lawniczek M, Kracker H, Sprenger T (2021) Ponesimod compared with teriflunomide in patients with relapsing multiple sclerosis in the active-comparator phase 3 OPTIMUM study: a randomized clinical trial. JAMA Neurol 78:558–567. https://doi.org/10.1001/jamaneurol.2021.0405
Guevara C, Garrido C, Martinez M, Farias GA, Orellana P, Soruco W, Alarcon P, Diaz V, Silva C, Kempton MJ, Barker G, de Grazia J (2019) Prospective assessment of no evidence of disease activity-4 status in early disease stages of multiple sclerosis in routine clinical practice. Front Neurol 10:788. https://doi.org/10.3389/fneur.2019.00788
Cree BAC, Mares J, Hartung H-P (2019) Current therapeutic landscape in multiple sclerosis: an evolving treatment paradigm. Curr Opin Neurol 32:365–377. https://doi.org/10.1097/WCO.0000000000000700
Tintore M, Rovira À, Río J, Otero-Romero S, Arrambide G, Tur C, Comabella M, Nos C, Arévalo MJ, Negrotto L, Galán I, Vidal-Jordana A, Castilló J, Palavra F, Simon E, Mitjana R, Auger C, Sastre-Garriga J, Montalban X (2015) Defining high, medium and low impact prognostic factors for developing multiple sclerosis. Brain 138:1863–1874. https://doi.org/10.1093/brain/awv105
Abdelhak A, Huss A, Kassubek J, Tumani H, Otto M (2018) Serum GFAP as a biomarker for disease severity in multiple sclerosis. Sci Rep 8:14798. https://doi.org/10.1038/s41598-018-33158-8
Acknowledgements
Medical writing support was provided by Rebecca Jarvis, PhD of Envision Pharma Group, Fairfield, CT, and was funded by Novartis Pharmaceuticals Corporation. This manuscript was developed in accordance with Good Publication Practice (GPP) guidelines. Authors had full control of the content and made the final decision on all aspects of this article.
Funding
This study was funded by Novartis Pharmaceuticals Corporation. Novartis Pharmaceuticals Corporation supported the development of this manuscript. Authors had full control of the content and made the final decision on all aspects of this publication.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and idea of the work, critically reviewed drafts of the manuscript, and approved the final version.
Corresponding author
Ethics declarations
Conflicts of interest
DO has received research support from Genentech, Genzyme, National Institutes of Health, National Multiple Sclerosis Society, Novartis, Patient-Centered Outcomes Research Institute, and Race to Erase MS Foundation; and has received consulting fees from Biogen, Genentech/Roche, Genzyme, Janssen, Merck, Novartis, and Pipeline Therapeutics. TC has received consulting fees from Genentech, Novartis, Sanofi Genzyme, Siemens; has received speaking fees from PRIME Education, LLC; and has received research support from BrainStorm Cell Therapeutics, Bristol Myers Squibb, Genentech/Roche, I-Mab Biopharma, National Institutes of Health, National Multiple Sclerosis Society, Novartis, Octave Bioscience, Sanofi Genzyme, Sumaira Foundation, Tiziana Life Sciences, and US Department of Defense. KR has received speaking, consulting, and advisory fees from Alexion Pharmaceuticals, Biogen, EMD Serono, Genentech, Genzyme, Horizon Therapeutics, Novartis, and TG Therapeutics; and has served as the principal investigator or co-investigator for grants to his institution from Alexion Pharmaceuticals, Biogen, EMD Serono, Genentech, Novartis, Sanofi Genzyme, and TG Therapeutics. AZO has received personal compensation for participation in scientific advisory boards, steering committees, and/or for speaking engagements from Alexion Pharmaceuticals, Banner Life Sciences, BD Biosciences, Biogen, Biologix Solutions, Bristol Myers Squibb, Celgene, EMD Serono, Genentech, GW Pharmaceuticals, Horizon Therapeutics, Jazz Pharmaceuticals, Novartis (local and global), Sandoz, Sanofi Genzyme, TG Therapeutics, and Viela Bio; has received consulting fee for serving as a scientific reviewer for the 2020 and 2022 Multiple Sclerosis Research Program (MSRP) for the Department of Defense Congressionally Directed Medical Research Programs (CDMRP); has received honoraria from Medscape, MJH Life Sciences, and WebMD; has served as a site principal investigator for studies funded (directly paid to Medical College of Wisconsin) by Atara Biotherapeutics, Biogen, Bristol Myers Squibb, Celgene, CorEvitas, EMD Serono, Genentech, GW Pharmaceuticals, Immunic Therapeutics, National Multiple Sclerosis Society/Patient-Centered Outcomes Research Institute, Novartis, Sanofi Genzyme, and TScan Therapeutics; and has received research funds from Central for Immunology, Clinical & Translational Science Institute (CTSI), National Institutes of Health, Neuroscience Research Center, and Research Affairs Committee.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ontaneda, D., Chitnis, T., Rammohan, K. et al. Identification and management of subclinical disease activity in early multiple sclerosis: a review. J Neurol 271, 1497–1514 (2024). https://doi.org/10.1007/s00415-023-12021-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-12021-5