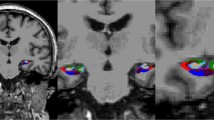
Abstract
While neurodegenerative and vascular neurocognitive disorder (NCD) often co-occur, the contribution of vascular lesions, especially stroke lesions identified on MRI, to global cognition in a real-life memory clinic population remains unclear. The main objective of this retrospective study was to determine NCD neuroimaging correlates: the GM atrophy pattern and vascular lesions (especially stroke lesion localization by voxel-based lesion-symptom mapping, VLSM) in a memory clinic. We included 336 patients with mild or major NCD who underwent cerebral MRI and a neuropsychological assessment. The GM atrophy pattern (obtained by voxel-based morphometry, VBM) and the stroke lesion localization (obtained by VLSM) associated with G5 z-score (a global cognitive score), were included as independent variables with other neuroimaging and clinical indices in a stepwise linear regression model. The mean age was 70.3 years and the mean MMSE score 21.3. On MRI, 75 patients had at least one stroke lesion. The G 5 z-score was associated with GM density in the pattern selected by the VBM analysis (R2 variation = 0.166, p < 0.001) and the presence of a stroke lesion in the region selected by the VSLM analysis (mainly in the right frontal region; R2 variation = 0.018, p = 0.008). The interaction between the two factors was insignificant (p = 0.374). In conclusion, in this first study combining VBM and VLSM analysis in a memory clinic, global cognition was associated with a specific GM atrophy pattern and the presence of a stroke lesion mainly in the right frontal region.




Similar content being viewed by others
Data availability
The conditions of our ethics approval do not permit public archiving of full data. Readers seeking access to full data should contact the corresponding author (DA) at the Department of Neurology, University of Picardy. Access will be granted to named individuals in accordance with ethical procedures governing the reuse of sensitive data.
References
Neuropathology Group. Medical Research Council Cognitive Function and Aging Study (2001) Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). Lancet 357:169–175. https://doi.org/10.1016/s0140-6736(00)03589-3
van der Flier WM, Scheltens P (2005) Epidemiology and risk factors of dementia. J Neurol Neurosurg Psychiatry 76:v2–v7. https://doi.org/10.1136/jnnp.2005.082867
Picard C, Pasquier F, Martinaud O et al (2011) Early onset dementia: characteristics in a large cohort from academic memory clinics. Alzheimer Dis Assoc Disord 25:203–205. https://doi.org/10.1097/WAD.0b013e3182056be7
Duyckaerts C, Bennecib M, Grignon Y et al (1997) Modeling the relation between neurofibrillary tangles and intellectual status. Neurobiol Aging 18:267–273
Seab JP, Jagust WJ, Wong ST et al (1988) Quantitative NMR measurements of hippocampal atrophy in Alzheimer’s disease. Magn Reson Med 8:200–208
Dickerson BC, Bakkour A, Salat DH et al (2009) The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex 19:497–510. https://doi.org/10.1093/cercor/bhn113
Besson FL, La Joie R, Doeuvre L et al (2015) Cognitive and brain profiles associated with current neuroimaging biomarkers of preclinical Alzheimer’s disease. J Neurosci 35:10402–10411. https://doi.org/10.1523/JNEUROSCI.0150-15.2015
Alexander SK, Rittman T, Xuereb JH et al (2014) Validation of the new consensus criteria for the diagnosis of corticobasal degeneration. J Neurol Neurosurg Psychiatr 85:925–929. https://doi.org/10.1136/jnnp-2013-307035
Boxer AL, Yu J-T, Golbe LI et al (2017) Advances in progressive supranuclear palsy: new diagnostic criteria, biomarkers, and therapeutic approaches. The Lancet Neurology 16:552–563. https://doi.org/10.1016/S1474-4422(17)30157-6
Gorno-Tempini ML, Hillis AE, Weintraub S et al (2011) Classification of primary progressive aphasia and its variants. Neurology 76:1006–1014. https://doi.org/10.1212/WNL.0b013e31821103e6
McKeith IG, Boeve BF, Dickson DW et al (2017) Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 89:88–100. https://doi.org/10.1212/WNL.0000000000004058
Rascovsky K, Hodges JR, Knopman D et al (2011) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134:2456–2477. https://doi.org/10.1093/brain/awr179
Yang J, Pan P, Song W et al (2012) Voxelwise meta-analysis of gray matter anomalies in Alzheimer’s disease and mild cognitive impairment using anatomic likelihood estimation. J Neurol Sci 316:21–29. https://doi.org/10.1016/j.jns.2012.02.010
Meeter LH, Kaat LD, Rohrer JD, van Swieten JC (2017) Imaging and fluid biomarkers in frontotemporal dementia. Nat Rev Neurol 13:406–419. https://doi.org/10.1038/nrneurol.2017.75
Ossenkoppele R, Pijnenburg YAL, Perry DC et al (2015) The behavioural/dysexecutive variant of Alzheimer’s disease: clinical, neuroimaging and pathological features. Brain 138:2732–2749. https://doi.org/10.1093/brain/awv191
Harper L, Bouwman F, Burton EJ et al (2017) Patterns of atrophy in pathologically confirmed dementias: a voxelwise analysis. J Neurol Neurosurg Psychiatry 88:908–916. https://doi.org/10.1136/jnnp-2016-314978
Sachdev P, Kalaria R, O’Brien J et al (2014) Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis Assoc Disord 28:206–218. https://doi.org/10.1097/WAD.0000000000000034
Puy L, Barbay M, Roussel M et al (2018) Neuroimaging determinants of poststroke cognitive performance: the GRECogVASC study. Stroke 49:2666–2673. https://doi.org/10.1161/STROKEAHA.118.021981
Weaver NA, Zhao L, Biesbroek JM et al (2019) The Meta VCI Map consortium for meta-analyses on strategic lesion locations for vascular cognitive impairment using lesion-symptom mapping: Design and multicenter pilot study. Alzheimer’s Dementia Diagnosis Assessment Disease Monit 11:310–326. https://doi.org/10.1016/j.dadm.2019.02.007
Weaver NA, Kuijf HJ, Aben HP et al (2021) Strategic infarct locations for post-stroke cognitive impairment: a pooled analysis of individual patient data from 12 acute ischaemic stroke cohorts. Lancet Neurol 20:448–459. https://doi.org/10.1016/S1474-4422(21)00060-0
White L, Small BJ, Petrovitch H et al (2005) Recent clinical-pathologic research on the causes of dementia in late life: update from the Honolulu-Asia aging study. J Geriatr Psychiatry Neurol 18:224–227. https://doi.org/10.1177/0891988705281872
Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA (2009) The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol 66:200–208. https://doi.org/10.1002/ana.21706
Kapasi A, DeCarli C, Schneider JA (2017) Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol 134:171–186. https://doi.org/10.1007/s00401-017-1717-7
Oveisgharan S, Dawe RJ, Yu L et al (2022) Frequency and underlying pathology of pure vascular cognitive impairment. JAMA Neurol 79:1277. https://doi.org/10.1001/jamaneurol.2022.3472
Toledo JB, Arnold SE, Raible K et al (2013) Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain 136:2697–2706. https://doi.org/10.1093/brain/awt188
Gladman JT, Corriveau RA, Debette S et al (2019) Vascular contributions to cognitive impairment and dementia: research consortia that focus on etiology and treatable targets to lessen the burden of dementia worldwide. Alzheimer’s Dementia Transl Res Clin Interv 5:789–796. https://doi.org/10.1016/j.trci.2019.09.017
Gorelick PB, Scuteri A, Black SE et al (2011) Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42:2672–2713. https://doi.org/10.1161/STR.0b013e3182299496
Sweeney MD, Montagne A, Sagare AP et al (2019) Vascular dysfunction—the disregarded partner of Alzheimer’s disease. Alzheimers Dement 15:158–167. https://doi.org/10.1016/j.jalz.2018.07.222
Heinen R, Groeneveld ON, Barkhof F et al (2020) Small vessel disease lesion type and brain atrophy: the role of co-occurring amyloid. Alzheimers Dement (Amst) 12:e12060. https://doi.org/10.1002/dad2.12060
Boomsma JMF, Exalto LG, Barkhof F et al (2017) Vascular cognitive impairment in a memory clinic population: rationale and design of the “Utrecht-Amsterdam Clinical Features and Prognosis in Vascular Cognitive Impairment” (TRACE-VCI) study. JMIR Res Protoc 6:e60. https://doi.org/10.2196/resprot.6864
Association American Psychiatric (2013) Diagnostic and statistical manual of mental disorders: DSM-5, 5th Revised. American Psychiatric Publishing, Washington
Albert MS, DeKosky ST, Dickson D et al (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:270–279. https://doi.org/10.1016/j.jalz.2011.03.008
McKhann GM, Knopman DS, Chertkow H et al (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:263–269. https://doi.org/10.1016/j.jalz.2011.03.005
Nelson PT, Dickson DW, Trojanowski JQ et al (2019) Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain 142:1503–1527. https://doi.org/10.1093/brain/awz099
Höglinger GU, Respondek G, Stamelou M et al (2017) Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord 32:853–864. https://doi.org/10.1002/mds.26987
Oslin D, Atkinson RM, Smith DM, Hendrie H (1998) Alcohol related dementia: proposed clinical criteria. Int J Geriatr Psychiatry 13:203–212
Nakajima M, Yamada S, Miyajima M et al (2021) Guidelines for management of idiopathic normal pressure hydrocephalus (third edition): endorsed by the Japanese Society of normal pressure hydrocephalus. Neurol Med Chir (Tokyo) 61:63–97. https://doi.org/10.2176/nmc.st.2020-0292
Dubois B, Feldman HH, Jacova C et al (2014) Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol 13:614–629. https://doi.org/10.1016/S1474-4422(14)70090-0
Godefroy O, Leclercq C, Roussel M et al (2012) French adaptation of the vascular cognitive impairment harmonization standards: the GRECOG-VASC study. Int J Stroke 7:362–363. https://doi.org/10.1111/j.1747-4949.2012.00794.x
Kalafat M, Hugonot-Diener L, Poitrenaud J (2003) Standardisation et étalonnage français du Mini Mental State (MMS) version GRÉCO. Rev Neuropsychol 13:209–236
Thuillard Colombo F, Assal G (1992) Adaptation française du test de dénomination de Boston. Versions abrégées. Eur Rev Appl Psychol 42:67–73
Deloche G, Hannequin D (1997) Test de dénomination orale d’images: DO 80. Éd. du Centre de psychologie appliquée, Paris, France
Albert ML (1973) A simple test of visual neglect. Neurology 23:658–664
Rey A (1959) Test de copie d’une figure complexe: Manuel., Les éditions du Centre de Psychologie Appliquée. Paris
Van der Linden M, Coyette F, Poitrenaud J, et les membres du GREMEM (2004) L’épreuve de rappel libre/rappel indicé à 16 items (RL/RI-16)"L’évaluation des troubles de la mémoire. Présentation de quatre tests de mémoire épisodique". Solal, Marseille
Baddeley A, Emslie H, Nimmo-Smith I (1994) Doors and people: a test of visual and verbal recall and recognition. Thames Valley Test Company, Suffolk
Wechsler D, Grégoire J (2000) Echelle d’Intelligence de Wechsler pour adultes: manuel : WAIS-III. Les Editions du Centre de Psychologie Appliquée, Paris
Godefroy O, Azouvi P, Robert P et al (2010) Dysexecutive syndrome: diagnostic criteria and validation study. Ann Neurol 68:855–864. https://doi.org/10.1002/ana.22117
Cardebat D, Doyon B, Puel M et al (1990) Formal and semantic lexical evocation in normal subjects. Performance and dynamics of production as a function of sex, age and educational level. Acta Neurol Belg 90:207–217
Reitan R (1958) Validity of trail making tests as an indicator of organic brain damage, Percept Mot skill
Godefroy O, Grefex (Groupe de Réflexion pour l’évaluation des Fonctions EXécutives) (2008) Fonctions exécutives et pathologies neurologiques et psychiatriques: évaluation en pratique clinique, Solal. Marseille
Montgomery SA, Asberg M (1979) A new depression scale designed to be sensitive to change. Br J Psychiatry 134:382–389
Lewinsohn PM, Seeley JR, Roberts RE, Allen NB (1997) Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging 12:277–287
Goldberg D, Bridges K, Duncan-Jones P, Grayson D (1988) Detecting anxiety and depression in general medical settings. BMJ 297:897–899
Lawton MP, Brody EM (1969) Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 9:179–186
Godefroy O, Gibbons L, Diouf M et al (2014) Validation of an integrated method for determining cognitive ability: Implications for routine assessments and clinical trials. Cortex 54:51–62. https://doi.org/10.1016/j.cortex.2014.01.016
Roussel M, Godefroy O (2016) La batterie GRECOGVASC : Evaluation et diagnostic des troubles neurocognitifs vasculaires avec ou sans contexte d’accident vasculaire cérébral. DE BOECK UNIVERSITE
Box GEP, Cox DR (1964) An analysis of transformations. Journal of the Royal Statistical Society Series B (Methodological 211–252
Dufouil C, Dubois B, Vellas B, et al (2017) Cognitive and imaging markers in non-demented subjects attending a memory clinic: study design and baseline findings of the MEMENTO cohort. Alzheimer’s Res Ther. https://doi.org/10.1186/s13195-017-0288-0
Godefroy O, Duhamel A, Leclerc X, et al (1998) Brain–behaviour relationships. Some models and related statistical procedures for the study of brain-damaged patients. Brain 121(Pt 8):1545–1556
Hachinski V, Iadecola C, Petersen RC et al (2006) National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke 37:2220–2241. https://doi.org/10.1161/01.STR.0000237236.88823.47
Cordonnier C, Potter GM, Jackson CA et al (2009) improving interrater agreement about brain microbleeds: development of the Brain Observer MicroBleed Scale (BOMBS). Stroke 40:94–99. https://doi.org/10.1161/STROKEAHA.108.526996
Wardlaw JM, Smith EE, Biessels GJ et al (2013) Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 12:822–838. https://doi.org/10.1016/S1474-4422(13)70124-8
Fazekas F, Chawluk JB, Alavi A et al (1987) MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol 149:351–356. https://doi.org/10.2214/ajr.149.2.351
Wahlund LO, Barkhof F, Fazekas F et al (2001) A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke 32:1318–1322. https://doi.org/10.1161/01.str.32.6.1318
Scheltens P, Leys D, Barkhof F et al (1992) Atrophy of medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatr 55:967–972
Charidimou A, Linn J, Vernooij MW et al (2015) Cortical superficial siderosis: detection and clinical significance in cerebral amyloid angiopathy and related conditions. Brain 138:2126–2139. https://doi.org/10.1093/brain/awv162
Doubal FN, MacLullich AMJ, Ferguson KJ et al (2010) Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke 41:450–454. https://doi.org/10.1161/STROKEAHA.109.564914
Schmidt P, Gaser C, Arsic M et al (2012) An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis. Neuroimage 59:3774–3783. https://doi.org/10.1016/j.neuroimage.2011.11.032
Ashburner J, Friston KJ (2005) Unified segmentation. Neuroimage 26:839–851. https://doi.org/10.1016/j.neuroimage.2005.02.018
Resnick SM, Pham DL, Kraut MA et al (2003) Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci 23:3295–3301
Tzourio-Mazoyer N, Landeau B, Papathanassiou D et al (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289. https://doi.org/10.1006/nimg.2001.0978
Arnoux A, Toba MN, Duering M et al (2018) Is VLSM a valid tool for determining the functional anatomy of the brain? Usefulness of additional Bayesian network analysis. Neuropsychologia 121:69–78. https://doi.org/10.1016/j.neuropsychologia.2018.10.003
Arnoux A, Triquenot-Bagan A, Andriuta D et al (2017) Imaging characteristics of venous parenchymal abnormalities. Stroke 48:3258–3265. https://doi.org/10.1161/STROKEAHA.117.017937
Catani M, Thiebaut de Schotten M (2008) A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 44:1105–1132. https://doi.org/10.1016/j.cortex.2008.05.004
Vermeer SE, Prins ND, den Heijer T et al (2003) Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 348:1215–1222. https://doi.org/10.1056/NEJMoa022066
Olsson Y, Brun A, Englund E (1996) Fundamental pathological lesions in vascular dementia. Acta Neurol Scand 94:31–38. https://doi.org/10.1111/j.1600-0404.1996.tb00370.x
Knopman DS, Parisi JE, Boeve BF et al (2003) Vascular dementia in a population-based autopsy study. Arch Neurol 60:569–575. https://doi.org/10.1001/archneur.60.4.569
Ferrer I (2010) Cognitive impairment of vascular origin: neuropathology of cognitive impairment of vascular origin. J Neurol Sci 299:139–149. https://doi.org/10.1016/j.jns.2010.08.039
Ishii K, Kawaguchi T, Shimada K et al (2008) Voxel-based analysis of gray matter and CSF space in idiopathic normal pressure hydrocephalus. Dement Geriatr Cogn Disord 25:329–335. https://doi.org/10.1159/000119521
Syaifullah AH, Shiino A, Fujiyoshi A et al (2021) Alcohol drinking and brain morphometry in apparently healthy community-dwelling Japanese men. Alcohol 90:57–65. https://doi.org/10.1016/j.alcohol.2020.11.006
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259
Braak H, Braak E (1995) Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging 16:271–278; discussion 278–284
Ozzoude M, Ramirez J, Raamana PR et al (2020) Cortical Thickness estimation in individuals with cerebral small vessel disease, focal atrophy, and chronic stroke lesions. Front Neurosci 14:598868. https://doi.org/10.3389/fnins.2020.598868
Debette S, Markus HS (2010) The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 341:c3666. https://doi.org/10.1136/bmj.c3666
Jacobs HIL, Visser PJ, Van Boxtel MPJ et al (2012) Association between white matter hyperintensities and executive decline in mild cognitive impairment is network dependent. Neurobiol Aging 33:201.e1–8. https://doi.org/10.1016/j.neurobiolaging.2010.07.015
Choe YM, Baek H, Choi HJ et al (2022) Association between enlarged perivascular spaces and cognition in a memory clinic population. Neurology. https://doi.org/10.1212/WNL.0000000000200910.10.1212/WNL.0000000000200910
Godefroy O, Spagnolo S, Roussel M, Boucart M (2010) Stroke and action slowing: mechanisms, determinants and prognosis value. Cerebrovasc Dis 29:508–514. https://doi.org/10.1159/000297968
Roussel M, Martinaud O, Hénon H, et al (2016) The behavioral and cognitive executive disorders of stroke: the GREFEX Study. PLoS One. https://doi.org/10.1371/journal.pone.0147602
Author information
Authors and Affiliations
Contributions
Daniela Andriuta: data acquisition, data and image analysis, statistics, and first draft of the manuscript. Emmanuel Wiener: data acquisition, image analysis, and revision of the manuscript. Alexandre Perron: data acquisition, image analysis, and revision of the manuscript. Elisa Ouin: data acquisition, image analysis, and revision of the manuscript. Ines Masmoudi: data acquisition and revision of the manuscript. William Thibaut: image analysis and revision of the manuscript. Jeanne Martin: image analysis and revision of the manuscript. Martine Roussel: data acquisition, data analysis, and revision of the manuscript. Jean-Marc Constans: image acquisition and revision of the manuscript. Ardalan Aarabi: revision of the manuscript. Olivier Godefroy: data and image analysis, statistics, study design, and revision of the manuscript. All co-authors agree with the conditions noted on the Authorship Agreement Form.
Corresponding author
Ethics declarations
Conflicts of interest
The authors report no disclosures of relevance to the manuscript. Daniela Andriuta reports funding (travel and meetings) from Biogen, Roche, Teva, Novartis, Bristol-Myers Squibb, Genzyme, and Sanofi outside the submitted work. Emmanuel Wiener reports no disclosures. Alexandre Perron reports funding (travel and meetings) from Biogen, Roche, Teva, Novartis, Bristol-Myers Squibb, Genzyme, and Sanofi outside the submitted work. Elisa Ouin reports no disclosures. Ines Masmoudi reports funding (travel, meetings and boards) funding Biogen, Roche, Teva, Novartis, Bristol-Myers Squibb, Genzyme, Merck, Janssen, Pfizer and Sanofi. William Thibaut reports no disclosures. Jeanne Martin reports fundings (travel, meetings and fees payed for service to a company) from LFB biomédicaments, Biogen, UCB Pharma SA, and Jazz Pharmaceuticals outside the submitted work. Martine Roussel reports no disclosures. Jean-Marc Constans reports no disclosures. Ardalan Aarabi reports no disclosures. Olivier Godefroy reports no disclosures.
Ethical approval
Ethical approval was obtained from the local institutional review board (CNIL: N° PI2020-843-0144). The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Andriuta, D., Wiener, E., Perron, A. et al. Neuroimaging determinants of cognitive impairment in the memory clinic: how important is the vascular burden?. J Neurol 271, 504–518 (2024). https://doi.org/10.1007/s00415-023-12009-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-12009-1