Abstract
Background
Epilepsy is a chronic brain disease characterized by recurrent seizures. We investigated real-world management of epilepsy across treatment lines in Spain, including healthcare resource use (HRU) and associated costs.
Methods
This was a retrospective study of real-life data from epilepsy patients prescribed antiseizure medication (ASM) between January 2016 and December 2021. Patients were grouped according to their line of treatment (1st, 2nd, 3rd and 4th +) during the recruitment period. Demographic and clinical characteristics, comorbidities and concomitant medications were analyzed during the baseline period (6 months before starting treatment line); antiepileptic treatments, concomitant medications, HRU and associated costs were analyzed during follow-up.
Results
The study included 5006 patients. Treatment duration decreased as treatment lines progressed (mean ± SD progression time: 523.2 ± 279.1 days from 1st to 2nd line, 351.6 ± 194.4 days from 2nd to 3rd line; 272.7 ± 139.3 days from 3rd to 4th + line). Significant HRU differences were found with subsequent treatment lines, including an increase in hospital admissions and patients on sick leave. Mean (95% CI) adjusted total costs per patient were €2974/year (2773–3175) in the 1st line and €5735/year (5043–6428) in the 4th + line. There was an increase in adjusted direct and total costs with subsequent treatment lines; the mean difference in total costs between cohorts was €2761 (p < 0.001). The highest direct costs were associated with epilepsy medication, days at the hospital and specialist visits.
Conclusion
Our data revealed a progressive increase in the use of resources and associated costs across subsequent epilepsy treatment lines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epilepsy is a chronic brain disease characterized by recurrent seizures and is one of the most common neurologic diseases, with approximately 50 million people affected worldwide [1, 2]. The annual incidence rate of epilepsy has been estimated at 50.4 to 81.7 cases per 100,000 individuals [1, 3], and according to the Global Burden of Disease Study, epilepsy constitutes a significant cause of disability and mortality [4]. In Spain, the mortality rate of patients with epilepsy is estimated to be two to three times higher than that of the general population [5]. In addition, patients with epilepsy present with many comorbidities, such as depression, anxiety, dementia, and migraine [6].
The ultimate epilepsy treatment goals are to achieve seizure freedom without clinically significant adverse effects and to improve quality of life [5, 7, 8]. Epilepsy guidelines recommend a first-line treatment based on monotherapy, followed by a second monotherapy or a first adjuvant treatment if seizures persist [5]. Nonetheless, existing guidelines do not contain therapeutic algorithms recommending the use of specific pharmacological alternatives for each treatment line [5, 8, 10].
Drug-resistant epilepsy (DRE) is defined by the International League Against Epilepsy (ILAE) as the failure of two tolerated, appropriately chosen and used antiepileptic drug schedules (whether as monotherapies or in combination) to achieve sustained seizure freedom [6, 11]. In recent decades, the number of antiseizure medications (ASMs) has increased and many therapeutic alternatives are now available [5], but studies suggest that 30–40% of patients can still be defined as drug-resistant based on the ILAE definition [12,13,14]. Poor medication adherence and comorbidities are key predictors of lack of seizure control [15].
The costs associated with epilepsy management depend on disease duration and severity, response to treatment, and healthcare setting [16,17,18]. Consequently, patients with poor control and more severe forms of epilepsy, such as DRE, use more healthcare resources, resulting in substantial costs for healthcare systems [17,18,19]. In addition, epilepsy causes significant productivity losses derived from the higher frequency of unemployment and precariousness among patients [16, 20]. Despite the existing evidence regarding the clinical and economic implications of epilepsy, data on the management of patients across treatment lines is limited [21]. In Spain, real-life studies regarding epilepsy management, healthcare resource use, and associated costs for the Spanish National Health System (SNHS) and society are scarce. This 6-year, retrospective study aimed to analyze the clinical and economic consequences of the current management of adult patients with epilepsy across treatment lines in Spain using clinical practice data from a large administrative database collected between January 2016 and December 2021. To that end, we studied: the demographic characteristics and comorbidities of patients with epilepsy across treatment lines; the epilepsy treatments and concomitant medications used in clinical practice; and the use of healthcare resources and associated costs.
Methods
Study design
This was a retrospective, observational study to assess clinical and economic consequences of current epilepsy management across treatment lines in Spain. Data from adult patients with epilepsy who started an ASM between January 2016 and December 2021 (recruitment period) were obtained from electronic medical records (EMRs) collated within the BIG-PAC® administrative database.
BIG-PAC® is a dissociated and anonymous administrative database. It contains the integrated records of GP visits (primary care), emergency care, pharmacy dispensements/prescriptions (verified daily dose record, time interval, and duration of each treatment administered), hospital admissions, working days lost and disability data, and deaths data collected since 2012 from the computerized medical records of seven integrated public health areas of Spain covering 1.9 million patients. BIG-PAC® is registered with the European Network of Centers for Pharmacoepidemiology and Pharmacovigilance with dependency of the European Medicines Agency (EMA) and has shown representativeness of the Spanish population [22, 23]. Before exporting to BIG-PAC®, primary data collected in EMRs were anonymized at the center of origin, in compliance with Organic Law 3/2018 of December 5 on the Protection of Personal Data and guarantee of digital rights [24].
The study was approved by the Ethics Committee of the Consorci Sanitari of Terrassa. Furthermore, it was developed following the ethical principles originating from the latest version of the Declaration of Helsinki accepted by local authorities and which are in line with Good Clinical Practice (GCP) and the requirements of current Spanish regulations. This study followed the requirements of the Reporting of studies Conducted using Observational Routinely-collected health Data (RECORD) [25].
Inclusion and exclusion criteria
Inclusion criteria were: (a) 18 years old or older; (b) epilepsy diagnosis (defined according to the International Classification of Disease, 9th Revision, Clinical Modification [ICD-9-CM] code: 345 [26]); (c) being active patient in the database for a minimum of 12 months before starting the study; (d) inclusion in the chronic prescription program to obtain medical prescriptions (with a verified record of the daily dose, the time interval, and the duration of each treatment administered; ≥ 2 prescriptions during the follow-up period); having a regular follow up with ≥ 2 health records in the system computer. Exclusion criteria were: (a) transference to other centers; (b) relocation; (c) being permanently institutionalized; and (d) suffering a terminal disease and/or being treated with dialysis.
Study cohorts
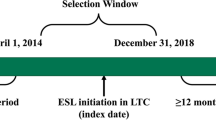
The study population was divided into four groups according to the number of ASMs that they had been prescribed at the index date, as follows: cohort 1, patients on first-line treatment; cohort 2, patients on second-line treatment; cohort 3, patients on third-line treatment; and cohort 4, patients on fourth line (or above) treatment (Fig. 1). The index date was defined as the date of initiation of the last treatment line received by the patient within the recruitment period. Patients were followed up from the index date until December 2021. The EMRs of selected patients were also retrospectively reviewed to assess the comorbidities and concomitant medications of the patient during the baseline period (6 months before the index date).
Study diagram. *Only treatments that lasted more than 3 months were considered to establish the sequence of treatment. Stratification by treatment was carried out in all patients recruited in the study. If, for instance, a patient was treated with 1 ASM, then with 2 ASMs, and finally with 3 ASMs during the recruitment period, the index date for that patient would be the date when 3 ASMs were prescribed and the cohort in which that patient was included for the analysis would be the cohort of patients treated with 3 ASMs. ASM, antiseizure medication; HRU, health resource use
Study endpoints
Study endpoints included demographic characteristics, comorbidities, treatment information, and healthcare resource use and costs.
Baseline parameters
Demographic variables (age and sex) were recorded at the index date. Comorbidities registered within six months before the index date were recorded using the ICD-9-CM coding system [26] (Table S1), including hypertension, diabetes, dyslipidemia, obesity, active smoking, alcohol ingestion, ischemic heart disease, cerebrovascular accident, heart failure, kidney failure, asthma, chronic obstructive pulmonary disease, depressive syndrome, and malignant neoplasms. Other comorbidities, such as anxiety, psychoses, and attention deficit disorder with hyperactivity, were also considered. In addition, the Charlson comorbidity index [27] was calculated to obtain a general comorbidity composite variable and a proxy to severity (Table S2).
Treatment information
Treatment information was obtained from drug dispensing records. Physicians chose drugs for each specific patient at their discretion, according to clinical practice. Drugs were collected using the Anatomical Therapeutic Chemical (ATC) Classification System [28] as follows: antiepileptics (N03), psycholeptics (N05; including antipsychotics, anxiolytics, hypnotics and sedatives), and psychoanaleptics (N06; including antidepressants and stimulants). Treatment durations of less than three months were considered as withdrawn. Therefore, only antiepileptic treatments lasting more than three months were considered to establish a treatment sequence. The main ASMs considered are summarized in Table 3. Concomitant medications considered in the analysis are shown in Table 4.
Healthcare resource use and costs
Healthcare resource use included visits to primary care, emergency services and specialists (including psychiatry, psychology and neurology), hospitalizations (annual rate and length of stay [days]), laboratory and diagnostic tests (conventional radiology, computed tomography, nuclear magnetic resonance, and electroencephalography), and medication. Hospitalizations also included admissions for surgical procedures, as described in Table S3. The healthcare resources consumed during the follow-up period were normalized per patient and year.
Healthcare costs (i.e., direct costs) were calculated considering the frequency of use during the follow-up and their unit cost for 2020 (based on hospital accounting) (Table S4). Medical prescriptions were quantified according to the retail price per pack at the time of prescription [29]. Non-healthcare costs (i.e., indirect costs) included those associated with productivity loss (i.e., absenteeism) and were measured as cost per day of sick leave due to temporary or permanent disability in the working population (< 65 years), considering the number of sick leave days/permanent disability days and the mean salary of the Spanish population, according to the National Institute of Statistics (Instituto Nacional de Estadística. n.d.) (Table S4).
Statistical analysis
SQL scripts were used for BIG-PAC data extraction. The data were carefully reviewed through exploratory analysis and preparation of data for analysis by observing the frequency distributions and searching for possible recording or coding errors. Data validation was carried out to ensure the quality of the results. SQL and MS Access were used for data processing and statistical analysis, including data collection, retrieval, and preparation procedures. Qualitative variables were described using absolute and relative frequencies (N, %), and quantitative variables using the mean and standard deviation (SD), median and interquartile ranges (IQR: P25–P75; Q1–Q3), and confidence intervals of 95% (95% CI). Bivariate analyses were performed using analysis of variance (ANOVA), Chi-square tests, and Pearson linear correlation. Analysis of covariance (ANCOVA) was used to adjust healthcare, indirect, and total costs (i.e., dependent variables) to covariates, including age, sex, and the Charlson index. Statistical significance was set at a two-sided α = 0.05. All statistical analyses were performed using the SPSSWIN program version 27.
Results
Demographic characteristics and comorbidities
A total of 5006 patients with epilepsy meeting the inclusion criteria were included and categorized into four groups (i.e., cohorts) according to epilepsy treatment line (Fig. 2). Patients were followed up until death, loss of follow-up or end of the recruitment period (31/12/2021) with a mean (± SD) follow-up time of 3.4 ± 1.6 years in cohort 1, 3.2 ± 1.6 years in cohort 2, 3.2 ± 1.6 years in cohort 3, and 3.1 ± 1.5 years in cohort 4. The characteristics of the study population stratified by treatment line are summarized in Table 1. Most patients were in 1st and 2nd-line treatments (86.7%), and the percentages progressively decreased throughout treatment lines (Fig. 3). Patients were similarly distributed across treatment lines regarding sex and age, with more than half of patients between 18 and 44 years old. The frequencies of comorbidities associated with epilepsy were similar among cohorts, except for depressive syndrome, which was more frequent among patients in the 4th + -line treatment. Hypertension, dyslipidemia, and depressive syndrome were the most frequent comorbidities. Overall morbidity analyses show that the mean number of diagnoses (p = 0.037) and the mean Charlson index value (p < 0.001) were significantly different across treatment lines, with an increasingly higher Charlson index mean throughout treatment lines. Cohort 4 had a higher percentage of patients with a high Charlson index score (i.e., ≥ 3), with no significant differences between cohorts.
Treatment patterns and duration
The distribution of the main antiepileptic drugs prescribed in the study cohorts is summarized in Table 2. In cohort 1, the most frequently used drugs in monotherapy were levetiracetam (28.6%), valproic acid (20.2%), and carbamazepine (10%). In cohort 2, more than 80% of patients received polytherapy, with the most frequently used combinations being levetiracetam + valproic acid (27.5%) or + eslicarbazepine (18.2%) or + carbamazepine (10%). The most frequently used drugs in monotherapy in cohort 2 were levetiracetam (3.9%), valproic acid 3.1%, and lacosamide (2.4%). In cohort 3, less than 10% of patients received monotherapy; the most frequent combinations were levetiracetam + valproic acid + oxcarbazepine (24.3%), levetiracetam + valproic acid + lamotrigine (18.2%), and levetiracetam + valproic acid + perampanel (15.7%). In cohort 4, 100% of the patients received polytherapy; the most frequently used combinations were levetiracetam + valproic acid + oxcarbazepine + zonisamide (37.6%) and levetiracetam + valproic acid + lamotrigine + clonazepam (32.1%).
The duration of epilepsy treatments was calculated from the index date up to the end of follow-up. Treatment duration decreased as treatment lines progressed. The mean (± SD) progression time was 523.2 ± 279.1 days from the 1st to the 2nd treatment line, 351.6 ± 194.4 days from the 2nd to the 3rd line, and 272.7 ± 139.3 days from the 3rd to the 4th + treatment line (Table 3).
Concomitant medications were analyzed at baseline (during the 6 months prior to index date) and significant differences were found in the administration of psycholeptics between cohorts (p = 0.057). Over 65% of the total population used these drugs, reaching a 70.9% use in cohort 4 (Table 4). However, the administration of psychoanaleptics and concomitant medications was similar among cohorts. The most prescribed concomitant medications were drugs for acid-related disorders, anti-inflammatories, antirheumatics, and lipid-modifying agents (Table 4).
Use of healthcare resources and associated costs
Significant resource use differences among cohorts were found in all the variables analyzed except for computed tomography (Table 5). An increase in hospital admissions and patients on sick leave was observed across treatment lines.
Consistent with the use of resources pattern, the associated costs per patient and year were significantly different among cohorts, showing a progressive increase throughout treatment lines. Treatment cohort 1 showed the lowest costs, with mean (± SD) direct costs of €2414/year (± 4394) and indirect costs of €556.1/year (± 2333), generating a total estimated cost of €2970/per patient and year (± 5111). Treatment cohort 4 showed the highest costs, with direct costs of €4903/year (± 4967), indirect costs of €831.1/year (± 2277), and a total cost of €5734/per patient and year (± 5837) (Table 6). Evaluation of costs adjusted by age, sex, and Charlson index confirmed the increase in direct and total costs throughout treatment lines, with a mean difference of €2761 in total costs between cohorts (p < 0.001). The mean adjusted total costs were €2974 (95% CI 2773–3175) in cohort 1, and €5735 (95% CI 5043–6428) in cohort 4 (Fig. 4). However, no statistically significant differences in indirect costs were found among cohorts. The highest direct costs were epilepsy medication (overall mean cost of €959.6/year [± 2704]), followed by days at the hospital (overall mean cost of €552.9/year [± 2710]), and specialist visits (overall mean cost of €425.1/year [± 1220]).
Discussion
This study provides real-world evidence on the characteristics, comorbidities and most frequently used treatments of patients with epilepsy in Spain. We also evaluated the duration of the therapy lines, and the progression of healthcare resource use and associated costs across epilepsy treatment lines.
Study cohorts were defined according to treatment line and our results show that the patients’ characteristics were comparable among them. Comorbidities were similar among cohorts when individually compared, except that depressive syndrome was more prevalent in cohort 4. However, when the Charlson Index, a weighted index to predict risk of death for patients with specific comorbid conditions was calculated, a trend towards a higher comorbidity burden was observed with treatment line progression, with a significantly higher score in cohort 4. This agrees with the previously described higher comorbidity burden in patients with medically refractory epilepsy [30].
Previous studies reported that approximately a third of patients remain uncontrolled after treatment with at least two antiepileptic drugs [12,13,14]. In our study, the proportion of patients in the ≥ 3rd line of treatment was 13.3%. However, in cohorts 1 and 2 of our study, a high proportion of patients had a follow up that was shorter than the average time to progress to the next line of treatment. Therefore, DRE could not be ruled out in these patients.
Patients with newly diagnosed focal epilepsy have been shown to have a higher prevalence of mood disorders, anxiety disorders and suicidality than the general population [31]. In addition, studies have shown an association between DRE and depressive disorders [32, 33]. In agreement, our results showed that 30.8% of the patients in the 4th + treatment line, in which 93.2% of the patients were prescribed ≥ 3 antiepileptic drugs, were diagnosed with depressive disorder as compared with 22.6%, 21.3% and 23.4% in the 1st, 2nd and 3rd treatment lines, respectively. For patients with DRE, depression is a significant predictor of reduced quality of life [34]. Therefore, special attention must be given to prevent depressive disorder during the management of patients with epilepsy, not only in those with DRE but also in patients with newly diagnosed disease.
Analysis of the main ASMs prescribed during the study period revealed a broad heterogeneity in drug combinations used from the second line onwards, in line with a lack of a specific therapeutic algorithm. Many patients were treated with levetiracetam, in accordance with a previous study [35]. We also observed widespread use of the first-generation drug valproic acid. This might be due to its high effectiveness against generalized seizure and related epileptic syndromes [36], despite the restrictions in women with childbearing potential [37]. For third-generation drugs such as brivaracetam, which have only become available in Spain more recently, prescriptions recorded in our study may be lower than current usage based on the time period of the study. We also observed scarce use of valproate + lamotrigine in the 3rd and ≥4th lines of therapy compared with other polytherapies, despite this combination being shown to be effective in the management of patients with refractory epilepsy based on the synergistic effect of the two agents [38].
Our data show a decrease in treatment duration as treatment lines progressed. This could be due to the perception of poor disease control by the physician, whose alternative might be moving the patient to the following treatment line. This reduction in treatment line duration was accompanied by an increase in HRU use and associated costs. Our results corroborate that poor control of epilepsy involves substantial costs; the incremental cost between lines 1 and 2 was smaller than between lines 2 and 3, which in turn was smaller than between lines 3 and 4, thus indicating that costs progressively increased across treatment lines. Moreover, the cost difference widened with each line (the difference between lines 3 and 4 was bigger than between lines 1 and 2). In this regard, other studies found that DRE was associated with increased expenditure. Villanueva et al. described that the patients diagnosed with DRE had higher direct epilepsy-related costs than non-DRE patients [18]. Similarly, Willems et al. found that, compared with non-DRE, DRE entailed higher expenditure in terms of total, direct, and indirect costs of illness [39]. Furthermore, Zelicourt et al. reported that the use of almost all healthcare resources was higher in patients with DRE; consequently, the direct epilepsy-related costs were more than double that in non-DRE patients [40]. Similarly, DRE costs in Spanish daily clinical practice have been shown to be associated with lower patient-reported outcome scores, highlighting how the increasing negative impact of DRE on the patient leads to higher costs [41].
The mean annual direct cost per patient of €2803 found in our study is consistent with that reported by other authors (range €1698–5432 per patient and year) [20, 39, 42, 43]. In addition, the estimated mean annual direct cost per patient was €3829 and €4903 for patients receiving 3rd and ≥4th lines of treatment, respectively, which are in line with those found in other studies (range €3777–6304 per patient and year) [18, 40, 44]. Direct medical costs increased with the treatment line and the main component of these direct costs was pharmacological treatment. This agrees with previously published data showing that the number of antiepileptic drugs, seizure frequency, and disease duration are significantly associated with the cost of illness of epilepsy [45]. In contrast with the recommendations of epilepsy guidelines [5, 9, 10], our data shows that 533 out of 662 patients in 3rd and ≥4th line therapy (85.7%) were treated with 3–4 antiepileptic medications, increasing the direct costs associated with the management of the disease. In addition, the number and duration of hospitalizations also increased with treatment line, which also explains the increase in economic burden in subsequent treatment lines.
The potential confounding effect of age, sex, and comorbidities on direct and indirect costs was addressed with an adjusted analysis. The difference in total and direct costs among cohorts remained statistically significant after adjustment, but the difference in indirect costs did not. In this regard, our analysis of indirect costs did not include unemployment or early retirement, which have been described as major contributors to indirect costs [39, 43, 46, 47]. Furthermore, indirect costs among cohorts were adjusted for comorbidities, including depression, even though depressive disorders are known to be associated with DRE [32, 33] and with an increased risk of sick leave [48], which in turn has an impact on epilepsy-associated indirect costs. In our study, the prevalence of depressive syndrome increased significantly in patients with more complex disease and associated advanced lines of treatment. Even after adjusting for depression, there was a noticeable trend toward increased indirect costs with later lines of therapy. Together, our direct and indirect cost data indicate that delaying epilepsy control increases the burden of epilepsy for the healthcare system and society. While little progress has been made to improve seizure-free rates among patients in the last few decades, use of new ASMs at earlier lines of therapy may provide an opportunity to improve seizure control [49], thereby reducing costs. Notably, new ASMs such as cenomabate (for focal seizures) and fenfluramine (for developmental and epileptic encephalopathy) were not commercialized in Spain at the time of database closure (December 2021) and are not therefore factored into our analysis.
This study has some limitations. First, the BIG-PAC® database is administrative and may lack some data about the study population, especially if patients have been treated in private healthcare centers or in public healthcare centers that are out of the scope of BIG-PAC®. The missing data may lead to classification bias and errors in categorizing diseases and the operational extent of costs. Another limitation is that the classification of patients by pathology in the BIG-PAC® database is based on the ICD-9-CM coding system and not on the ILAE classification. Moreover, our study did not include direct non-healthcare costs (i.e., out-of-pocket costs or those paid for by the patient/family) as they are not registered in the database and the study design does not provide direct access to patients. Another significant limitation is the lack of information about the reasons for treatment change, which has prevented us from discriminating treatment failures from other reasons for treatment change and, therefore, calculating the proportion of DRE patients. Moreover, we have no information on how many patients are treated in epilepsy referral centers, which might impact on clinical practice. However, there are several strengths as this study included a large sample of patients with epilepsy, allowing us to capture valuable data on the public Spanish National Health System and to analyze the use of resources and costs by treatment line. In addition, we assessed not only direct, healthcare-related costs but also indirect costs associated with productivity loss. Although the differences between healthcare systems may hinder the application of our results to other settings, this analysis highlights the importance of early control of epilepsy to reduce the use of resources and the costs associated with this disease.
Conclusions
In conclusion, this study confirmed the substantial expenditure and use of resources derived from managing patients with epilepsy and found a progressive increase in the use of resources and costs across subsequent treatment lines. Consequently, the early control of epilepsy may not only benefit patients but also reduce the economic burden for healthcare providers.
Data availability
All of the data used for this study is available from BIG-PAC®, a dissociated and anonymized administrative database; the secondary data used in this study are not linked to patients’ identity and may be shared at the request of any qualified investigator for purposes of replicating procedures and results.
References
Falco-Walter J (2020) Epilepsy—definition, classification, pathophysiology, and epidemiology. Semin Neurol 40:617–623
World Health Organization (WHO). Epilepsy [Internet]. 2021. https://www.who.int/news-room/fact-sheets/detail/epilepsy. Accessed 12 Nov 2021
Valencia-Calderón C, Rumià-Arboix J, Conesa-Bertrán G et al (2021) Estado actual de la cirugía de la epilepsia en España. Compendio y conciencia Revista de Neurología 72:92–102
Beghi E, Giussani G, Nichols E et al (2016) Global, regional, and national burden of epilepsy, 1990–2016: a systematic analysis for the Global Burden of Disease Study. Lancet Neurol 18:357–375
Sociedad Española de Neurología. Manual de Práctica Clínica en Epilepsia. Recomendaciones diagnostico-terapéuticas de la SEN [Internet]. 2019. http://epilepsia.sen.es/wp-content/uploads/2020/06/Recomendaciones-Epilepsia-SEN-2019.pdf. Accessed 11 Nov 2021
Keezer MR, Sisodiya SM, Sander JW (2016) Comorbidities of epilepsy: current concepts and future perspectives. Lancet Neurol 15:106–115
Panayiotopoulos CP. The epilepsies: seizures, syndromes and management. Chapter 4, Principles of therapy in epilepsies. Available from: [Internet]. Oxfordshire (UK): Bladon Medical Publishing; 2005. https://www.ncbi.nlm.nih.gov/books/NBK2607/
Schachter SC (2005) Improving quality of life beyond seizure control. Epileptic Disord 7(Suppl 1):S34-38
American Epilepsy Society Guidelines [Internet]. Default. 2022. https://aesnet.org/clinical-care/epilepsy-self-management/other-self-management-information. Accessed 24 Mar 2023
Mercadé Cerdá JM, Toledo Argani M, Mauri Llerda JA et al (2016) Guía oficial de la Sociedad Española de Neurología de práctica clínica en epilepsia. Neurologia 31:121–129
Kwan P, Arzimanoglou A, Berg AT et al (2010) Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 51:1069–1077
Chen Z, Brodie MJ, Liew D et al (2018) Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: a 30-year longitudinal cohort study. JAMA Neurol 75:279–286
Golyala A, Kwan P (2017) Drug development for refractory epilepsy: the past 25 years and beyond. Seizure 44:147–156
Sultana B, Panzini MA, Veilleux Carpentier A et al (2021) Incidence and prevalence of drug-resistant epilepsy: a systematic review and meta-analysis. Neurology 96:805–817
Niriayo YL, Mamo A, Kassa TD et al (2018) Treatment outcome and associated factors among patients with epilepsy. Sci Rep 8:17354
Allers K, Essue BM, Hackett ML et al (2015) The economic impact of epilepsy: a systematic review. BMC Neurol 15:245
Quintana M, Fonseca E, Sánchez-López J et al (2021) The economic burden of newly diagnosed epilepsy in Spain. Epilepsy Behav 125:108395
Villanueva V, Girón JM, Martín J et al (2013) Impacto económico y en calidad de vida de la epilepsia resistente en España: estudio ESPERA. Neurologia 28:195–204
Villanueva V, Brosa M, Doz M et al (2011) PMH70 Economic impact of focal epilepsy in Spain: results of the Espera Study. Value Health 14:A299
Pato Pato A, Cebrián Pérez E, Cimas Hernando I et al (2011) Análisis de costes directos, indirectos e intangibles de la epilepsia. Neurologia 26:32–38
Perrone V, Veronesi C, Dovizio M et al (2022) Analysis of patients with focal epilepsy and drug-resistant epilepsy in Italy: Evaluation of their characteristics, therapeutic pathway and the consumption of healthcare resources. Clinicoecon Outcomes Res 14:513–521
Sicras-Mainar A, Enriquez JL, Hernández I et al (2019) PMU146 Validation and representativeness of the Spanish BIG-PAC database: integrated computerized medical records for research into epidemiology, medicines and health resource use (real world evidence). Value Health 22:S734
Sicras-Mainar A, Sicras-Navarro A, Palacios B et al (2020) Epidemiología y tratamiento de la insuficiencia cardiaca en España: estudio PATHWAYS-HF. Rev Esp Cardiol 149:240–247
Boletín Oficial del Estado. Ley Orgánica 15/1999, de 13 de diciembre, de Protección de Datos de Carácter Personal [Internet]. 1999 p. 43088–99. https://www.boe.es/buscar/doc.php?id=BOE-A-1999-23750
Benchimol EI, Smeeth L, Guttmann A et al (2015) The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med 12:e1001885
Ministerio de Sanidad, Consumo y Bienestar Social. International Classification of Diseases (9th edition) Clinical Modification (ICD-09-CM). [Internet]. 2021. https://eciemaps.mscbs.gob.es/ecieMaps/browser/index_9_mc.html. Accessed 11 Jun 2021
Deyo RA, Cherkin DC, Ciol MA (1992) Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45:613–619
World Health Organization (WHO). The anatomical therapeutic chemical classification system with defined daily doses (ATC/DDD) [Internet]. 2022. https://www.who.int/standards/classifications/other-classifications/the-anatomical-therapeutic-chemical-classification-system-with-defined-daily-doses. Accessed 8 Apr 2021
Consejo General de Colegios Oficiales de Farmacéuticos. BOT Plus 2. Base de Datos de Medicamentos [Internet]. https://botplusweb.portalfarma.com/. Accessed 13 Apr 2021
van Hezik-Wester V, de Groot S, Kanters T et al (2022) Burden of illness in people with medically refractory epilepsy who suffer from daily to weekly seizures: 12-month follow-up of participants in the EPISODE study. Front Neurol 13:1012486
Kanner AM, Saporta AS, Kim DH et al (2023) Mood and anxiety disorders and suicidality in patients with newly diagnosed focal epilepsy: an analysis of a complex comorbidity. Neurology 100:e1123–e1134
Nogueira MH, Yasuda CL, Coan AC et al (2017) Concurrent mood and anxiety disorders are associated with pharmacoresistant seizures in patients with MTLE. Epilepsia 58:1268–1276
Ribot R, Ouyang B, Kanner AM (2017) The impact of antidepressants on seizure frequency and depressive and anxiety disorders of patients with epilepsy: Is it worth investigating? Epilepsy Behav 70(Pt A):5–9
Boylan LS, Flint LA, Labovitz DL et al (2004) Depression but not seizure frequency predicts quality of life in treatment-resistant epilepsy. Neurology 62:258–261
Hochbaum M, Kienitz R, Rosenow F et al (2022) Trends in antiseizure medication prescription patterns among all adults, women, and older adults with epilepsy: a German longitudinal analysis from 2008 to 2020. Epilepsy Behavior 130:108666
Romoli M, Mazzocchetti P, D’Alonzo R et al (2019) Valproic acid and epilepsy: from molecular mechanisms to clinical evidences. Curr Neuropharmacol 17:926–946
European Medicines Agency (EMA). Valproate-related-substances; article-31-referral-prac-assessment-report_en.pdf [Internet]. 2014. https://www.ema.europa.eu/en/documents/referral/valproate-related-substances-article-31-referral-prac-assessment-report_en.pdf. Accessed 21 Apr 2023
Knoester PD, Keyser A, Renier WO et al (2005) Effectiveness of lamotrigine in clinical practice: results of a retrospective population-based study. Epilepsy Res 65:93–100
Willems LM, Hochbaum M, Frey K et al (2022) Multicenter, cross-sectional study of the costs of illness and cost-driving factors in adult patients with epilepsy. Epilepsia 63:904–918
de Zélicourt M, de Toffol B, Vespignani H et al (2014) Management of focal epilepsy in adults treated with polytherapy in France: the direct cost of drug resistance (ESPERA study). Seizure 23:349–356
Peña P, Sancho J, Rufo M et al (2009) Driving cost factors in adult outpatients with refractory epilepsy: a daily clinical practice in clinics of neurology in Spain. Epilepsy Res 83:133–143
Noda AH, Hermsen A, Berkenfeld R et al (2015) Evaluation of costs of epilepsy using an electronic practice management software in Germany. Seizure 26:49–55
Pirker S, Graef A, Gächter M et al (2021) Costs of epilepsy in Austria: unemployment as a primary driving factor. Seizure 89:24–29
Guekht A, Mizinova M, Kaimovsky I et al (2016) The direct costs of epilepsy in Russia. A prospective cost-of-illness study from a single center in Moscow. Epilepsy Behav 64(Pt A):122–126
Melkamu P, Animut Y, Minyihun A et al (2021) Cost of illness of epilepsy and associated factors in patients attending adult outpatient department of University of Gondar Referral Hospital, Northwest Ethiopia. Risk Manag Healthc Policy 14:2385–2394
Hamer HM, Spottke A, Aletsee C et al (2006) Direct and indirect costs of refractory epilepsy in a tertiary epilepsy center in Germany. Epilepsia 47:2165–2172
Strzelczyk A, Reese JP, Oertel WH et al (2013) Costs of epilepsy and their predictors: cross-sectional study in Germany and review of literature. Epileptology 1:55–60
Amiri S, Behnezhad S (2021) Depression symptoms and risk of sick leave: a systematic review and meta-analysis. Int Arch Occup Environ Health 94:1495–1512
Klein P, Krauss GL, Steinhoff BJ et al (2023) Failure to use new breakthrough treatments for epilepsy. Epilepsia 64:1458–1465
Acknowledgements
The authors thank Ignacio Hernández (Atrys Health) and Ian Marshall (WriteMedical Ltd) for editorial assistance.
Funding
The study was funded by Angelini Pharma.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
RT has received honoraria and/or research funds from Angelini Pharma, Bial, Eisai, Esteve, Jazz Pharmaceuticals and UCB Pharma. VV has received honoraria and/or research funds from Angelini Pharma, Bial, Biocodex, Eisai, Jazz Pharmaceuticals, Neuraxpharm, Novartis, Nutricia, Takeda, UCB Pharma and Xenon. MT has received honoraria and/or research funds from Angelini Pharma, Bial, Eisai, Esteve, Jazz Pharmaceuticals, Krka, Neuraxpharm, Sanofi-Aventis and UCB Pharma. PPD and JS are employees of Angelini Pharma.
Ethical standards
The study was approved by the Ethics Committee of the Consorci Sanitari of Terrassa and performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. As BIG-PAC® is a dissociated and anonymized administrative database, the secondary data used in this study were not linked to patients’ identity and no informed consent was required.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Toledano, R., Villanueva, V., Toledo, M. et al. Clinical and economic implications of epilepsy management across treatment lines in Spain: a real-life database analysis. J Neurol 270, 5945–5957 (2023). https://doi.org/10.1007/s00415-023-11958-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-11958-x