Abstract
Much has changed since our last review of recent advances in neuro-otology 7 years ago. Unfortunately there are still not many practising neuro-otologists, so that most patients with vestibular problems need, in the first instance, to be evaluated and treated by neurologists whose special expertise is not neuro-otology. The areas we consider here are mostly those that almost any neurologist should be able to start managing: acute spontaneous vertigo in the Emergency Room—is it vestibular neuritis or posterior circulation stroke; recurrent spontaneous vertigo in the office—is it vestibular migraine or Meniere's disease and the most common vestibular problem of all—benign positional vertigo. Finally we consider the future: long-term vestibular monitoring and the impact of machine learning on vestibular diagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The patient with recurrent acute vertigo attacks
Amongst patients seen by appointment, complaining of what after careful interrogation sounds like recurrent acute vertigo attacks, the differential diagnosis is basically limited to benign positional vertigo (BPV), Meniere’s disease (MD) or vestibular migraine (VM) [1]. Patients who start to have posterior circulation transient ischemic attacks with predominant vertigo will usually have a stroke long before their appointment comes around [2].
Benign positional vertigo (BPV)
BPV is the commonest cause of recurrent vertigo; it presents as brief spins lasting seconds, triggered by bending down, looking up or rolling over in bed [3, 4]. The elderly might present with falls after getting out of bed [5]. BPV is caused by otoconia dislodged from one of the otolith membranes moving under the influence of gravity, either within a semicircular canal (SCC) duct itself (“canalithiasis”) [6, 7] or while attached to its cupula (“cupulolithiasis”). As the head moves from one position to another with respect to gravity, the otoconia move and increase or decrease the resting activity of canal afferents [8], producing vertigo and a nystagmus with its rotation axis orthogonal to the plane of the stimulated canal [9]. Careful clinical observation and analysis of the exact beating direction (i.e. rotation axis) of this position-provoked nystagmus [10] and of the exact provocative position, allows deductions to be made about which SCC in which ear is being stimulated in which direction—towards the ampulla which is excitatory for the lateral SCC but inhibitory for the vertical SSCs, or away from the ampulla which is inhibitory for the lateral SCC but excitatory for the vertical SCCs. These deductions then guide repositioning manoeuvres. Informative simulations of the presumed movement of the otoconia in the SCCs have been produced [11,12,13,14,15]. A useful collection of BPV videos, uploaded by Dr Dan Gold, can be found on the University of Utah, Neuro-ophthalmology Virtual Education Library (NOVEL) website [16].
Posterior canal BPV
Typical posterior semicircular canal (PSC) BPV accounts for almost 90% of all BPV presentations [17]. The Dix-Hallpike test produces almost immediate geotropic-torsional and upbeating vertical nystagmus indicating that the otoconia are moving in the excitatory direction, that is away from the PSC cupula in the lowermost ear (Fig. 1). (Note: The term “geotropic” when applied to positional nystagmus means that the quick phases of the nystagmus beat towards the lowermost ear; “apogeotropic” means towards the uppermost ear.) Diagnostic criteria for typical posterior canal BPV [18] require: (1) recurrent attacks of positional vertigo or dizziness provoked by lying down or turning over while supine; (2) attack duration of < 1 min; (3) positional nystagmus elicited after a latency of a few seconds by the Dix-Hallpike or the side-lying manoeuvre; (4) geotropic torsional, vertical upbeating (PSC plane) nystagmus lasting < 1 min and (5) that no other disorder better accounts for these findings [3, 4]. Investigations are indicated only when an underlying cause for BPV is suspected [19].
The typical nystagmus profile of right posterior canal BPV. When the subject is upright (A), no nystagmus is seen. In the right Hallpike position (B), after a latency of 2–3 s, a paroxysm of upbeating torsional geotropic nystagmus is seen, with a crescendo-decrescendo vertical slow phase velocity (SPV) profile
Horizontal canal BPV
Typical horizontal semicircular canal (HSC) BPV—also known as lateral semicircular canal BPV—accounts for about 10% of all BPV presentations. There are several variants, all with some type of horizontal positional nystagmus. Three examples follow. (A) Paroxysmal horizontal nystagmus beating towards the lowermost ear (i.e. geotropic nystagmus). This is attributed to canalithiasis of the HSC in the ear that is lowermost when lying on the side with the higher nystagmus slow-phase velocity (Fig. 2). This nystagmus has a shorter onset latency than PSC-BPV, a crescendo-decrescendo pattern and a relatively longer duration, still less than 1 min [4, 9, 20]. (B) Persistent horizontal nystagmus beating towards the uppermost ear (i.e. apogeotropic nystagmus). This is attributed to cupulolithiasis of the HSC in the ear that is uppermost when lying on the side with the higher nystagmus slow-phase velocity. (C) Persistent horizontal geotropic nystagmus that is symmetrical to each side. This has been attributed to a “light cupula”, i.e. a cupula with a lower than normal specific gravity [21], but not everyone believes this [22]. Both geotropic and apogeotropic horizontal positional nystagmus have also been reported in vestibular migraine [20, 23, 24].
The nystagmus profile of right horizontal semicircular canal BPV. (A) When the subject is upright, there is no nystagmus. (B) When the affected right ear is placed lowermost, there is after a latency of ~ 1 s or less, a 35s paroxysm of geotropic (right-beating) horizontal nystagmus. It has a crescendo-decrescendo slow-phase velocity profile with a peak velocity of 83 deg/s. (C) When the unaffected left ear is placed lowermost there is a similar duration but less intense paroxysm of geotropic (left-beating) horizontal nystagmus. It also has a crescendo-decrescendo slow-phase velocity profile with a peak velocity of 35 deg/s
Treatment of BPV
Typical PSC-BPV can usually be treated effectively and immediately with an Epley or a Semont [25,26,27,28] manoeuvre by physiotherapists [29], audiologists [30] or doctors. Some patients learn to treat themselves [31], often by following one of many self-help BPV online videos. HSC-BPV can be harder to treat than PSC-BPV and many different repositioning manoeuvres are used; many are named after the neuro-otologist who first proposed it [28]. The simplest just has the patient lie only on the unaffected side for “as long as possible, preferably all night” [32]. On the basis of modelling, a universal BPV repositioning manoeuvre has been proposed [33]. Unfortunately, even now only a few of the many patients who present to an Emergency Room [34,35,36] or to a primary care clinic [37] with vertigo even have a Dix-Hallpike test correctly performed; most just have blood tests and brain CT and are prescribed useless anti-emetic tablets.
Mechanical rotators for treating BPV
There can be practical problems with treating even a simple case of unilateral PSC-BPV. For example, if the patient is 120 kg, 80 years old and has Parkinson's disease, it is impossible to do a proper Epley (or Semont) manoeuvre, or even an accurate Dix-Hallpike test, especially on a narrow examination couch jammed in the office corner up against a wall. Two solutions to this problem are: (1) a home visit: testing and treating the patients in their own home, on their own double bed, with their family helping; with video goggles [38] it is possible to check the nystagmus and to show sceptical family members that there really is something wrong with the patient. (2) Treating the patient in a mechanical repositioning device such as the Epley Omniax rotator (unfortunately no longer made) or the TRV chair, both motor-driven. These devices are suitable and effective [39] for diagnosing and treating patients with BPV that involves multiple canals or those with physical limitations (stroke, spine injuries, morbid obesity) that preclude effective bedside manoeuvres. A transportable manually operated device is also available [40].
Atypical BPV
There are many other patterns of positional nystagmus in patients who really do have peripheral positional vertigo (i.e. BPV) rather than central positional vertigo [41]. For example, in one type of atypical PSC-BPV the patient has apogeotropic torsional, downbeating nystagmus in the Dix-Hallpike position rather than geotropic, upbeating nystagmus. This could be taken to indicate anterior SCC BPV, but soon the patient develops nystagmus of typical PSC canalithiasis from the opposite side [17, 42]. These patients are thought to have otoconia in the distal part of the non-ampullary arm of the PSC, close to the common crus. Dix-Hallpike testing moves this mass towards the ampulla, thus inhibiting posterior canal afferents and producing an inhibitory torsional downbeating nystagmus. This positional nystagmus can be provoked in either right or left Dix-Hallpike positions, the head-hanging position and sometimes, even in a side lying position; there is a crescendo-decrescendo time-course but no latency and the nystagmus is not completely exhaustible. Rising to the upright position does not reverse nystagmus direction, and it does not fatigue on repeated positioning. Two treatments have been proposed: the second half of the Semont manoeuvre which the patient begins by sitting upright with legs hanging over the edge of the bed, the head rotated towards the healthy ear; then while maintaining this head position, lies onto the unaffected side, thus allowing the otoconia to fall into the common crus and finally the vestibule. The second treatment, termed the “45° forced prolonged position”, requires subjects to lie on the unaffected side with the head turned 45° downwards to bring the non-ampullary arm of the affected posterior canal into a draining position and to maintain this for eight hours [42]. Atypical BPV can be difficult to distinguish from central positional vertigo [43, 44] (see below), and in our view the diagnosis should be made by a neuro-otologist.
BPV after acute vestibular syndrome
When BPV accompanies or follows an acute vestibular syndrome, the cause of the acute vestibular syndrome should be confirmed with video head impulse testing (vHIT), vestibular evoked myogenic potentials (VEMPs) and audiometry. With BPV secondary to vestibular neuritis [45, 46] there can be impaired ocular VEMPs and horizontal plus anterior canal vHITs but normal cervical VEMPs [47]. In contrast, with BPV after labyrinthitis or labyrinthine infarct, there is also sudden hearing loss [48, 49], and there can be prolonged geotropic or apogeotropic positional nystagmus refractory to treatment (as in cupulolithiasis) and abnormal posterior canal vHIT [50, 51].
Positional vertigo without positional nystagmus
If the story sounds like BPV, but there is neither positional vertigo, nor positional nystagmus with a correctly done Dix-Hallpike test, it is best to see the patient again [52] rather than order tests. While an unequivocal diagnosis of BPV requires paroxysmal positional nystagmus, some patients who keep having positional vertigo but have no nystagmus during the Dix-Hallpike test can do just as well as those who do have nystagmus after a repositioning manoeuvre [53, 54]. Others only have vertigo after coming up from the Dix-Hallpike test but do have retropulsion and measurable oscillation of the trunk at the same time, possibly due to otoconia on the utricular side of the PSC. These patients can be treated effectively with repeated sit-ups from the Dix-Hallpike position, aimed at liberating otoconia from the short arm of the PSC [55].
Positional nystagmus without positional vertigo
With removal of visual fixation, an asymptomatic low-velocity (2–5°/s) positional nystagmus, horizontal or vertical of almost every conceivable kind, occurs in many (maybe even most) normal subjects—even in those without a history of BPV or migraine [56, 57]. This needs to be considered when a patient who seems to have had BPV, but is now in remission, has some positional nystagmus in the dark.
Central positional vertigo and nystagmus
Positional vertigo and positional nystagmus (paroxysmal, persistent or both) can be the presenting feature of some focal lesions and diffuse diseases affecting the cerebellum or the brainstem [58,59,60]. Downbeating nystagmus on straight head-hanging, upbeating nystagmus on returning to the upright position from supine and apogeotropic nystagmus during the supine head-roll test all occur in central paroxysmal positional nystagmus [58]. The direction of central paroxysmal positional nystagmus aligns with the vector sum of the rotational axes of the semicircular canals that were being inhibited in each position: for example, a straight head-hanging position would inhibit both anterior canals, and so the nystagmus is directly upbeat with no latency to onset and a rapid crescendo phase which decreases exponentially. Time constants for the nystagmus, 3–8 s, correspond to those of the vertical vestibulo-ocular reflex (VOR). The possibility of a central positional vertigo/nystagmus is particularly important to consider in a patient presenting without any other neurological symptoms or signs, just with atypical BPV [17, 41]. Could the cause of the positional vertigo be just migraine [23, 61, 62] or perhaps something more sinister such as a structural lesion? Unfortunately, not even a high-quality, contrast-enhanced MRI with thin, overlapping slices is totally reassuring, as the problem might be an MRI negative, antibody mediated, autoimmune process [63, 64].
Meniere’s disease
So, if it is not BPV, then is it MD or is it VM, or maybe both [65, 66]? There is a close relationship between the two [67], and some actually consider MD to be a vestibulo-cochlear subtype of migraine [68]. The diagnosis of MD is easy if there is unilateral tinnitus and aural fullness with a fluctuating, low-frequency, cochlear-type sensorineural hearing loss which might not be obvious during, or even after, the first few vertigo attacks, but will be eventually [69]. Moreover, the patient might be too dizzy during attacks to notice the hearing problem and could not in any case cooperate with an audiogram. There are smartphone apps offering pure-tone air-conducted audiograms with which it is possible to check if there is a temporary hearing loss with the vertigo attacks [70]. This way any reasonably tech-savvy patient should be able to do their own audiogram on a regular basis in between and just after vertigo attacks. There is no other cause of a low-frequency hearing loss that comes and goes (Fig. 3). Accurate audiological evaluation and interpretation by an audiologist, in cooperation with an otologist, is essential to make the diagnosis of MD. Diagnostic difficulties could arise if the patient has a pre-existing, unrelated hearing loss such as low-frequency conductive (otosclerosis), mid-frequency sensorineural (congenital) or high-frequency sensorineural (age/noise induced), or if the patient has bilateral MD. Drop attacks—in which the patient just drops to the ground—occur in MD as well as in some non-MD aural diseases, but not in migraine [71]. Unfortunately, while most neurologists will order an EEG and ECG in such patients, they will rarely order an audiogram [72]. Repeated attacks of Room Tilt Illusion—suddenly the whole visual world is tilted or even inverted for seconds or minutes—might be a related phenomenon: they can occur in both MD and migraine [73] and perhaps also with TIAs [74] or seizures [75]. Syncope, as a result of the strong vestibular sensation, is rare but potentially dangerous and easy to mistake for a drop attack in a patient with MD [76]. In between MD attacks, there will often be unilateral vestibular impairment of air-conducted ocular and cervical VEMPs and of caloric responses but not of the head impulse test [77,78,79,80]. Settings of the subjective visual horizontal (or vertical) might deviate, usually in the same direction as the slow phases of any spontaneous nystagmus [81]. During MD attacks, there is, almost invariably, horizontal nystagmus (sometimes with a vertical component) that can have a horizontal slow phase velocity over 160°/s. The nystagmus first beats towards the affected side (excitatory nystagmus), then towards the normal side (paretic nystagmus) and then again towards the affected side (recovery nystagmus) [82]. Without knowing from hearing loss which is the affected ear, the spontaneous nystagmus direction will not accurately lateralise the MD. This type of nystagmus is enhanced by head-shaking and skull vibration (apparently possible in certain stoical patients) [83]. Rarely, the video head impulse test is temporarily abnormal during an MD attack [84, 85] with either reduced [82, 86] or enhanced [87] responses from the lateral SCC. It is of interest that the VOR response to pulsed galvanic stimulation can also be enhanced in MD [88].
Three sequential pure-tone audiograms from the right ear of a 19-year-old female with Meniere's disease, showing the typical fluctuating, low-frequency, sensorineural hearing loss in the right ear. First audiogram is one month before a vertigo attack, second audiogram is one day after a vertigo attack, and the third two months after the attack. Compare with the normal audiogram from the unaffected left ear. The acoustic reflex thresholds—shown with star (*) symbols at the bottom of each graph—do not change with the increase in subjective pure-tone thresholds in the low frequencies, for example at 1kHz from 30 dB on 5 August to 60 dB on 16 September, indicating recruitment, which is considered characteristic of a cochlear hearing loss. Masked (m) bone conduction thresholds are shown with (<) symbols; there is no conductive component of the hearing loss
The vertigo attacks in MD can usually be stopped. Therapeutic total unilateral vestibular deafferentation of the affected ear with vestibular nerve section or labyrinthectomy [89] or partial deafferentation with intratympanic gentamicin [90] can do this, but at the risk of producing imbalance needing long-term vestibular rehabilitation [91,92,93], especially in the elderly [94]. Intratympanic dexamethasone might be just as good as gentamicin and will not produce imbalance [95]. A low-sodium diet is traditional [96], endolymphatic sac surgery controversial [97, 98], drugs such as betahistine [99, 100] or cinnarizine plus dimenhydrinate [101] still hopeful.
Vestibular migraine
Many patients with migraine headaches also have balance problems, including vertigo attacks [102,103,104,105,106,107], and many patients with vertigo attacks or other balance problems also have migraine headaches [61, 108, 109]. There are now official criteria for the diagnosis of VM [105, 110], even though many migraineurs have other, unofficial, balance problems [111] such as chronic subjective dizziness [112], motion sensitivity [113], motion sickness [114], constant rocking sensations (mal-de-debarquement) [115, 116], room-tilt illusion [73] or a generalised imbalance [117] which can respond to vestibular rehabilitation [118]. There are some characteristic differences between patients with VM and migraineurs without vestibular symptoms: a longstanding history of migraine with severe headache attacks, aural fullness/tinnitus accompanying attacks, presence of menopause and a history of motion sickness [107, 119]. There might be minor audiologic changes in VM [61, 120] but not a fluctuating, unilateral low-frequency hearing loss as in MD. Children have VM [121, 122]. (They also have BPV [123] but only rarely have MD [124].) Perhaps as a consequence of the vertigo attacks, some VM patients (and also some MD patients) develop psychological problems such as depression [125], anxiety [125,126,127], panic attacks [128], phobias [129] and of even more concern, possible cognitive impairment [130,131,132,133,134,135] which might however respond to therapy [136].
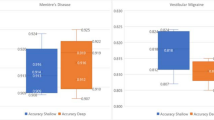
Between attacks VM patients can have some low-velocity spontaneous or positional nystagmus in darkness, usually horizontal and around 10°/s or less, but their vestibular function tests (vHIT, caloric and VEMP) are normal [61]. During a VM attack most have a direction-changing or direction-fixed spontaneous nystagmus [137], usually horizontal and less than 15°/s slow phase velocity (but sometimes up to 57°/s), or a persistent positional nystagmus [23] up to 100°/s slow phase velocity in 26% [61] (Fig. 4). Such ictal nystagmus in VM might need to be distinguished from the ictal nystagmus that can occur in MD [78, 82], central vestibulopathy [138] or BPV [139]. When patients have both MD and migraine then things get even more complicated [66, 140,141,142]. Also, patients can have headache with their BPV [143], and those who have migraine are more likely to have BPV than those who do not [144, 145].
Atypical positional nystagmus in a patient with clinically definite vestibular migraine. Sitting upright (A), there is left-beating horizontal spontaneous nystagmus, which persists with the left ear down (C), and reverses to right-beating with the right ear down (B). With either ear down, there is persistent horizontal geotropic nystagmus, which has a flat slow phase velocity (SPV) profile
Although there is no solid evidence of measurable benefit from treating or preventing VM [146,147,148,149], patients are of course treated [150], usually with drugs that are used for the treatment and prevention of migraine headaches [such as betablockers, pizotifen, tricyclics, anticonvulsants (topiramate, lamotrigine, valproate), cinnarizine, flunarizine or triptans] [109, 151, 152].
Differentiating MD from VM
In patients with recurrent acute spontaneous vertigo attacks that have been happening for more than say 3 months, MD and VM are the only two realistic diagnoses. If there is also unilateral tinnitus and aural fullness with a low frequency unilateral/asymmetrical sensorineural hearing loss, then it has to be MD. If there are no aural symptoms and no hearing loss, the differential diagnosis will hinge on the vestibular function tests. These should all be normal in VM, but in MD there may be: (1) a canal paresis > 25% on the caloric test with normal lateral SCC vHIT and (2) reduced air-conducted VEMPs, ocular and cervical, on the side with the caloric paresis. The spontaneous nystagmus seen during a vertigo attack is also useful for differentiating MD from VM [153] and is discussed below in our section on vestibular event monitoring.
Video head impulse testing
Short, fast, head accelerations (head impulses) test SCC afferents in much the same way as patellar tendon taps test 1a afferents. Head impulses test the vestibulo-ocular reflex in response to rapid (2000–3000°/s2) head accelerations. The VOR response to these fast stimuli is hard-wired into the neurophysiology of the SCCs and the brainstem; it depends on the resting rate and on–off asymmetry of primary SCC afferents and their robust direct disynaptic or trisynaptic excitatory and cross commissural inhibitory projections via the vestibular nuclei in the pons and medulla to the ocular motor nuclei in the pons and midbrain [154, 155]. The head impulse test [156], specifically the vHIT [157, 158], can detect moderate to severe impairment of any single SCC. It is sometimes (but not always) possible to detect this in the clinical HIT by noting the characteristic compensatory “catch-up” saccades [159,160,161]. The clinical head impulse test depends, as do other aspects of the neurological examination, on both the clinician’s skill and the patient’s co-operation. If the catch-up saccades have a short latency and so occur while the head is still moving rather than just after it has stopped moving, they will be “covert,” that is, invisible to the clinician but detectible on vHIT [158]. There are now three commercially available vHIT systems: two with goggle-based pupil-tracking cameras and one with a tripod-mounted camera [162]; each system has its strengths and weaknesses and potential pitfalls [163, 164]. With training and practice [165] neurologists, otolaryngologists, audiologists [166] and physiotherapists [167] can all now measure the VOR from each of the six SCCs in almost any reasonably co-operative adult [168] or child [169, 170] in about 20 min. Since 2016 when we wrote the previous version of this review, the yearly number of publications in PubMed dealing specifically with vHIT has increased from 62 to 186. Here, we consider four common clinical situations in which the vHIT could help with diagnosis.
vHIT during an acute vestibular syndrome
The patient is seen, usually in an Emergency Room, during her first-ever attack of acute, spontaneous, isolated vertigo. Assuming there is no simultaneous acute unilateral hearing loss (neurologists rarely ask about and almost never test for hearing loss), the two main diagnoses are vestibular neuritis and posterior circulation stroke involving the cerebellum and perhaps the brainstem vestibular nuclei. A competent, focused clinical examination which includes a head impulse test, such as HINTS [171, 172] or STANDING [173] can usually distinguish between the two. Videonystagmography plus vHIT [174,175,176,177] will double the rate of correct diagnosis [178]. In acute vestibular neuritis there is sudden unilateral loss of vestibular function [179]. All three SCCs might be involved or only the lateral and anterior which suggests involvement of only the superior vestibular nerve. A patient with left superior vestibular neuritis will have a horizontal/ torsional nystagmus beating to the right, more vigorously in right than in left gaze, suppressed by visual fixation and almost always a clinically obvious impairment of the left horizontal SCC VOR on the bedside HIT [161]. Here vHIT can provide objective, quantitative measures of the VOR from all six SCCs [180], documenting that there really is unilateral impairment of left lateral and anterior SCC function. Vestibular testing can be completed by finding a leftward offset of the subjective visual horizontal (or vertical), loss of left ocular VEMPs indicating impaired utricular function with intact cervical VEMPs [181, 182] indicating preserved saccular function [47, 156, 183] (Fig. 5). Selective inferior vestibular neuritis [157], affecting just the PSC, can only be confidently diagnosed with vHIT and corroborated by finding an absent cervical VEMP (from the saccule) and a preserved ocular VEMP (from the utricle) [184]. In contrast to acute vestibular neuritis, an acute cerebellar/brainstem infarct might not impair the VOR, so the patient will have a normal clinical HIT, a normal or near-normal vHIT [185,186,187], sometimes not even nystagmus [176], and may just complain of imbalance [188]. Here the logic is counter-intuitive: it is a normal test result, in this case the normal head impulse test (and no nystagmus), that indicates a potentially serious condition. Acute cerebellar infarction is not a diagnosis to miss [189], as there is chance of foramen magnum herniation needing immediate posterior fossa decompression [190] to prevent death or permanent disability [191], whereas an unequivocally abnormal test, the vHIT, indicates a potentially safe-to-discharge condition—vestibular neuritis. Two other conditions that can produce acute, isolated, spontaneous vertigo, MD and VM, also do not show impairment of the VOR on vHIT; they can be hard to differentiate from cerebellar infarction in the acute phase. However, it is unusual in MD for there not to be or have been unilateral tinnitus, fullness, and low-frequency hearing loss, even during the first attack—see above. On the other hand, patients with an MD vertigo attack are usually too busy being dizzy to complain about or even to notice a minor hearing problem, especially in the masking din of an Emergency Room and if nobody asks about it and if nobody can test for it. A severe, first-ever VM attack might be even more difficult to distinguish from cerebellar infarction—even by an experienced neuro-otologist. A combination of one, maybe even two, negative diffusion-weighted MRI scans [192] and a detailed headache history once the patient has recovered is probably the only way. The editor of Practical Neurology gives a clear and concise personal account of what it is like to have, and to have had, acute vestibular neuritis [193].
Vestibular neuritis. Vestibular test profile of a patient with left vestibular neuritis who presented with isolated acute spontaneous vertigo lasting three days. The vHIT shows reduced gain from the left horizontal (0.61) and anterior (0.63) semicircular canals with abnormal catch-up saccades. The ocular VEMP, indicating dynamic utricular function, is absent from the left ear, but cervical VEMPs, indicating dynamic saccular function, are symmetrical. cVEMP amplitudes divided by background rectified EMG activation show only a 6.6% asymmetry—abnormal in our laboratory is > 35%. The subjective visual horizontal, which tests the left–right balance of static utricular function, shows a very large (28°) counterclockwise (i.e. towards the left ear) offset indicating reduction in left utricular function. The audiogram shows only a mild, slightly asymmetrical (left > right) high-frequency hearing loss, almost certainly entirely unrelated to the vestibular neuritis
vHIT after an acute vestibular syndrome
The patient is seen days, weeks—whenever she can get an appointment—after such an attack. She might be asymptomatic and simply wants to know what happened and whether it could happen again. Or she might be complaining of persisting imbalance, because she really did have acute vestibular neuritis and while her brainstem has compensated [194], her peripheral vestibular function has not fully recovered and she now has chronic vestibular insufficiency [195], experiencing head movement oscillopsia and a feeling of imbalance with a positive foam Romberg test [196,197,198]. Or because she actually had a cerebellar infarct. Alternatively, she could be complaining of further, but less severe, vertigo attacks: if the attacks are spontaneous, she might actually have MD; if the attacks are positional, she might have PSC-BPV as a result of the vestibular neuritis [45, 46].
When a patient still has unilateral impairment of peripheral SCC function according to vHIT, caloric or rotational testing [196, 199,200,201] some weeks after the acute vestibular syndrome, the diagnosis of vestibular neuritis can be safely made in retrospect. If, however, peripheral vestibular function has largely recovered (with or without corticosteroids [202, 203]), the distinction between recovered peripheral (as opposed to centrally compensated) vestibular function [200, 204] and cerebellar infarction cannot be made clinically and will need MRI. If that too is normal, there is a diagnostic problem. Was this actually an MRI negative cerebellar infarct [192] or a cerebellar TIA [205] rather than a recovered vestibular neuritis or even post-stroke BPV [59]? Could the patient have had a cerebellar embolus from paroxysmal atrial fibrillation [206]? There are more questions than answers.
vHIT with recurrent vertigo attacks
The patient is seen when well, but complains of recurrent vertigo attacks, either spontaneous or positional. If the attacks really are vertigo, then VM, MD, and BPV are just about the only plausible diagnoses. Rarely, recurrent vertigo is cardiogenic [207]. Unfortunately, most patients who have started to have isolated vertigo attacks from vertebrobasilar TIAs will have a stroke long before their appointment comes around [2]. Recurrent vertigo attacks are the most common vestibular complaint in office practice, but vHIT rarely helps as it is usually normal inter-ictally, even in Meniere’s disease [77, 79, 80]. Nonetheless, it is still worth doing: occasionally BPV is secondary to some inner ear disease [19] and so in that case the vHIT could be abnormal. Posterior SCC vHIT might also be transiently abnormal due to canalithiasis itself [208, 209].
vHIT in chronic imbalance
There are many possible causes for a complaint of chronic imbalance: some neurological, such as sensory neuropathies, extrapyramidal disorders, orthostatic tremor, or normal pressure hydrocephalus, and others not, such as musculoskeletal disorders or mental health issues. What concerns us here is chronic vestibular insufficiency which can either be due to severe unilateral vestibular impairment [93, 195, 197, 210] or moderate to severe, symmetrical or asymmetrical, bilateral vestibular impairment [211,212,213]. The patient with chronic vestibular insufficiency might have no obvious symptoms while sitting or lying but feels imbalance as soon as she stands or walks. Despite this there might be little clinically obvious impairment of gait or of stance even with eyes closed and feet together—a negative Romberg test. But if the patient now tries to do a Romberg test on a soft surface, say a foam mat [214], then she will sway and could fall [215] if not caught. This is a positive foam Romberg test [198] which is almost diagnostic of vestibular impairment. Patients with proprioceptive impairment such as those with a hereditary neuropathy such as Charcot-Marie Tooth disease [216, 217] or chronic inflammatory demyelinating polyneuropathy [218] or a ganglionopathy such as CANVAS (cerebellar ataxia neuropathy vestibular areflexia syndrome) [219, 220] already have a positive Romberg test on the firm surface such as the floor but will be worse when standing on foam. Patients with bilateral vestibular impairment might also have difficulties with movement strategies, control of dynamics, orientation in space, and cognitive processing [221, 222]. Such patients will also notice vertical oscillopsia during rapid, passive vertical head-shaking [212] due to impairment of the vertical VOR. They might even volunteer, or at least admit, that they have to stop walking in order to see clearly. Having the examiner shake their head up-and-down will drop their vision by at least three lines on a Snellen chart. Bilateral vestibular impairment needs to be severe to be detectable on caloric or rotational tests, as these tests have large normal ranges. vHIT is the most reliable test to detect bilateral vestibular impairment [211, 213, 223, 224] as it has a tight age-adjusted normal range [225] and is even suitable for detecting age-related vestibular impairment, that is “presbyvestibulopathy” [226,227,228], also called “presbystasis” [229]. Although mild impairment of just one lateral SCC can be detected by caloric testing, it will not produce imbalance if it is only mild. vHIT is the best test for measuring whether vestibular function is by itself impaired sufficiently to produce imbalance. A possible cause of an isolated severe unilateral vestibular loss presenting with chronic vestibular impairment is an unrecognised previous attack of acute vestibular neuritis [230]; the patient might not have had or might not have noticed vertigo. If there is definitely no history of a previous vertigo attack, then a chronic progressive cause of unilateral vestibular loss such as a vestibular schwannoma (hearing should also be impaired) needs to be excluded [231,232,233]. The cause of non-syndromic bilateral vestibular impairment without hearing impairment usually remains undiagnosed unless it is bilateral sequential vestibular neuritis [234, 235], gentamicin toxicity [236], Wernicke’s encephalopathy [216] or maybe hereditary spastic paraplegia [237]. If accompanied by hearing impairment then other diagnoses need to be considered: hereditary disorders such as Usher syndrome [238, 239] and also acquired diseases such as superficial siderosis [240] and leptomeningeal carcinomatosis [241]. If there is also cerebellar impairment, as shown by an impaired visually enhanced VOR, then CANVAS [242] needs to be considered. If there is paradoxical enhancement of the VOR on vHIT, as well as of the visually enhanced VOR, then autosomal recessive cerebellar ataxia type 3 (ARCA3) which is due to a mutation in the ANO10 gene needs to be considered [243].
vHIT: potential practical pitfalls
Although vHIT can be quick and easy to do, it requires training, practice and attention to detail [163, 165, 168, 244]. For example, it is important to interact with the patient throughout testing, continually exhorting her to pay attention to the fixation target (as in visual field testing), not to blink, and not to resist or try to help with the passive head turning. It is important to give head impulse stimuli over the entire magnitude range up to 300°/s peak head velocity. Testing the vertical SCCs requires special attention to eccentric horizontal eye position [245]. The reason it is possible to test the three-dimensional vestibular sensory system with a two-dimensional method (the vHIT) is that when the eyes deviate horizontally so that they align with vertical impulses being delivered directly in a vertical SCC plane, then the VOR is entirely vertical; torsional eye movements, which cannot be detected by the video method, are eliminated. vHIT testing using a head-fixed rather than space-fixed visual target—the suppression Head Impulse (SHIMP) paradigm [246]—can give clearer results in patients with many covert saccades, especially those with only a little residual HSC function.
vHIT and caloric testing
The caloric has been the mainstay of vestibular testing for over a hundred years [247], and it still has a place in some cases with a normal lateral canal vHIT. It is now proposed that vHIT should be the first test done in a patient with a suspected vestibular problem [248, 249]. If the vHIT is abnormal, then there is no point in doing calorics—they will not give any more diagnostic information. If, however, the vHIT data are clean and truly normal over the entire stimulus magnitude range, then it might be worth asking for calorics [80]. For example, in MD the calorics might be impaired even when the vHIT is normal [79, 80, 166, 250]. One explanation for this discrepancy is that since MD preferentially causes impairment of type II vestibular hair cells [251], it will preferentially impair tonic HSC discharges (responsible for caloric responses) rather than phasic discharges (responsible for impulsive responses). Our alternative explanation, that the caloric impairment is a hydrodynamic effect from the swelling of the endolymphatic compartment abolishing the possibility of thermal convection [77]—the main proposed mechanism of caloric stimulation—is not supported by otopathologic studies [252]. Also, in patients with recovered vestibular neuritis, recovery might be less obvious on caloric testing than on vHIT, which means that a patient seen some time after an acute vestibular syndrome who now has a normal vHIT should have a caloric test—as it might still show a canal paresis [253], indicating that it really was vestibular neuritis rather than a brainstem/cerebellar stroke.
vHIT and VEMPs
VEMPs can give a semi-quantitative measurement of the function of each of the four otoliths—two utricles and two saccules [183, 184]. VEMPs combined with vHIT make it possible to test each of the 10 vestibular organs individually [254]. VEMPs are about as easy or difficult to do as any other evoked potential test in clinical neurophysiology. There are, however, some important specific technical details to follow in order to record meaningful VEMPs [183, 184]: (a) correct calibration of the air-conducted sound stimulus which needs to be loud enough to be effective but still safe [255, 256]; (b) an effective stimulator for bone-conducted VEMPs, such as a triggered tendon hammer or, for more accuracy, an electro-mechanical vibrator such as a Bruel & Kjaer minishaker; and (c) measurement of background rectified sternomastoid muscle EMG activation with cervical VEMP to make the left/right asymmetry ratio more accurate. What then are some clinical situations in which VEMP testing might be useful [257]? Consider the patient who is seen weeks after recovering from an acute vestibular syndrome who has no impairment of vHIT, but has a canal paresis on a caloric test [253]. Here, an absent ocular VEMP from ipsilateral utricle would confirm that the patient has had superior vestibular neuritis [181]. Similarly, if the patient has only an impaired posterior canal vHIT, then an absent cervical VEMP from the ipsilateral saccule could support the diagnosis of a previous inferior vestibular neuritis [47, 258]. VEMPs are particularly useful to help decide if a superior canal dehiscence shown on CT is symptomatic [259]: if the VEMP has a low threshold and a large amplitude, then it probably is [260,261,262].
Vestibular event monitoring
One of the difficulties when diagnosing patients with recurrent vertigo is that they are often asymptomatic when seen in the clinic. Patients with BPV, MD or VM have very mild or no nystagmus between attacks, but will often have marked spontaneous and/or positional nystagmus when symptomatic [61, 82]. This acute nystagmus has diagnostic value. For example, when differentiating MD and VM, spontaneous horizontal nystagmus with slow phase velocity > 12.05°/s during an attack is 82.1% specific for MD, whereas spontaneous vertical nystagmus is 93.0% sensitive for VM [153]. Devices have been developed which allow this nystagmus to be captured during a vertigo episode at home, either by patients self-recording using portable video goggles (Fig. 6) [153, 263] or a wearable electro-oculography device (CAVA) that provides continuous monitoring [264, 265]. Although these devices are not currently widely available—the DizzyDoctor System was marketed but support has recently been discontinued [263]—they will play an important role as a diagnostic aid in the near future. A similar problem applies to patients who are very vertiginous in the Emergency Room, but who are much less symptomatic when reviewed on the ward the next day or in the clinic several weeks later. Using video goggles to record acute nystagmus in the ER helps differentiate between stroke and vestibular neuritis, MD and VM, and BPV and central positional nystagmus [178].
Portable infra-red video goggles used for vestibular event monitoring (A), which were used by a patient with left-sided Meniere’s disease to self-capture at home sequential recordings from a single attack. The nystagmus profiles from the recordings (B) match the three phases of the classic direction-changing nystagmus seen during an attack of Meniere’s disease: excitatory nystagmus towards the affected side, then paretic nystagmus towards the normal side, and finally recovery nystagmus towards the affected side. Upward deflections indicate rightward eye movements, while downward deflections indicate leftward eye movements
Machine learning and vestibular diagnosis
In recent years, research into the applications of artificial intelligence and machine learning in healthcare has increased exponentially [266]. The field of neuro-otology has been no exception, and most of the efforts thus far explore the potential for machine learning tools to act as diagnostic decision aids [267]. In general terms, machine learning models take clinical data from patients with conditions of interest and apply various algorithms to ‘learn’ how to distinguish between the diagnoses, without needing to be explicitly programmed with rules to follow. Machine learning methods allow analysis of large, complex datasets including images, and can identify associations that are invisible to the human eye or traditional techniques. Unlike a clinician, their diagnostic performance is never affected by fatigue or carelessness.
The differential diagnosis of vertigo is a problem that is well suited to machine learning as there are often only a few plausible differentials, particularly within a specific vertigo syndrome. Machine learning methods have been applied to clinical data from history [268], examination findings including video eye recordings [269,270,271,272,273], vestibular function tests such as vHIT [274] and VEMPs [275], or a combination of the above [276,277,278]. Many studies report excellent model performance, including models achieving accuracies and/or area-under-the-curve scores of 0.95 or higher for distinguishing stroke from vestibular neuritis [279], VM or MD from other causes of dizziness [280], or between various subtypes of BPV [272]. However, such promising results come with caveats. Many models use data collected from a single site and have not yet been validated in populations with, for instance, diverse demographics or different laboratory setups. Furthermore, models are often trained using data carefully selected as being typical or free of artefact [272, 274], with exclusion of rarer conditions (such as cupulolithiasis or anterior canal BPV [270, 272]) or patients with unclear diagnoses [268]. Performance in real-world settings can be poorer than expected [281].
The immediate future of machine learning in neuro-otology lies in models which can be used in real-time by clinicians to assist diagnosis. Given the legal and regulatory complexities, it is likely to be some time before clinicians will be replaced by devices which can provide diagnoses autonomously. Emergency physicians, generalists and primary care physicians would be able to access tools that simulate the diagnostic expertise of the expert neuro-otologist and apply this to a much larger population of vertiginous patients. Ideally, models would use only a minimal number of input variables, so as to optimise clinical workflow and reduce computational power requirements, and would not rely heavily on technical expertise or specialised equipment. Promisingly, machine learning algorithms may be able to identify nystagmus even from low resolution images [271], as well as differentiate between common causes of vertigo using only information from a patient questionnaire [268]. Clinicians must familiarise themselves with the limitations of machine learning models and the risks associated with their use [282, 283], as the implementation of these technologies is inevitable.
References
Friedland DR, Tarima S, Erbe C, Miles A (2016) Development of a statistical model for the prediction of common vestibular diagnoses. JAMA Otolaryngol Head Neck Surg 142(4):351–356. https://doi.org/10.1001/jamaoto.2015.3663
Kim HA, Oh EH, Choi SY, Choi JH, Park JY, Lee H, Choi KD (2021) Transient vestibular symptoms preceding posterior circulation stroke: a prospective multicenter study. Stroke 52(6):e224–e228. https://doi.org/10.1161/STROKEAHA.120.032488
Kim JS, Zee DS (2014) Clinical practice. Benign paroxysmal positional vertigo. N Engl J Med 370(12):1138–1147. https://doi.org/10.1056/NEJMcp1309481
Argaet EC, Bradshaw AP, Welgampola MS (2019) Benign positional vertigo, its diagnosis, treatment and mimics. Clin Neurophysiol Pract 4:97–111. https://doi.org/10.1016/j.cnp.2019.03.001
van Dam VS, Maas BDPJ, Schermer TR, van Benthem PG, Bruintjes TD (2021) Two symptoms strongly suggest benign paroxysmal positional vertigo in a dizzy patient. Front Neurol 11:625776. https://doi.org/10.3389/fneur.2020.625776
Kao WT, Parnes LS, Chole RA (2017) Otoconia and otolithic membrane fragments within the posterior semicircular canal in benign paroxysmal positional vertigo. Laryngoscope 127(3):709–714. https://doi.org/10.1002/lary.26115
Nuti D, Zee DS, Mandalà M (2020) Benign paroxysmal positional vertigo: what we do and do not know. Semin Neurol 40(1):49–58. https://doi.org/10.1055/s-0039-3402733
Rajguru SM, Rabbitt RD (2007) Afferent responses during experimentally induced semicircular canalithiasis. J Neurophysiol 97(3):2355–2363. https://doi.org/10.1152/jn.01152.2006
Aw ST, Todd MJ, Aw GE, McGarvie LA, Halmagyi GM (2005) Benign positional nystagmus: a study of its three-dimensional spatio-temporal characteristics. Neurology 64(11):1897–1905. https://doi.org/10.1212/01.WNL.0000163545.57134.3D
Libonati GA, Martellucci S, Castellucci A, Malara P (2022) Minimum stimulus strategy: a step-by-step diagnostic approach to BPPV. J Neurol Sci 434:120158. https://doi.org/10.1016/j.jns.2022.120158
Bhandari A, Kingma H, Bhandari R (2021) BPPV Simulation: a powerful tool to understand and optimize the diagnostics and treatment of all possible variants of BPPV. Front Neurol 12:632286. https://doi.org/10.3389/fneur.2021.632286
Bhandari A, Bhandari R, Kingma H, Zuma e Maia F, Strupp M (2021) Three-dimensional simulations of six treatment maneuvers for horizontal canal benign paroxysmal positional vertigo canalithiasis. Eur J Neurol 28(12):4178–4183. https://doi.org/10.1111/ene.15044
Bhandari A, Bhandari R, Kingma H, Strupp M (2022) Modified interpretations of the supine roll test in horizontal canal BPPV based on simulations: how the initial position of the debris in the canal and the sequence of testing affects the direction of the nystagmus and the diagnosis. Front Neurol 13:881156. https://doi.org/10.3389/fneur.2022.881156
Teixido M, Woods O, Kung B, Seyyedi M (2016) A 3D benign paroxysmal positional vertigo model for study of otolith disease. World J Otorhinolaryngol Head Neck Surg 2(1):1–6. https://doi.org/10.1016/j.wjorl.2016.02.002
Teixido M, Traboulsi H, Osier P, Foster V, Northrop C, Levine S (2018) BPPV viewer. BPPV visualisation with a 3D model for study and teaching. https://bppvviewer.com. Accessed 29 June 2023
Gold D (2023) NOVEL—The Dan Gold Neuro-Ophthalmology Collection, University of Utah. https://novel.utah.edu/Gold/. Accessed 16 June 2023
Califano L, Mazzone S, Salafia F, Melillo MG, Manna G (2021) Less common forms of posterior canal benign paroxysmal positional vertigo. Acta Otorhinolaryngol Ital 41(3):255–262. https://doi.org/10.14639/0392-100X-N1032
von Brevern M, Bertholon P, Brandt T, Fife T, Imai T, Nuti D, Newman-Toker D (2015) Benign paroxysmal positional vertigo: diagnostic criteria. J Vestib Res 25(3–4):105–117. https://doi.org/10.3233/VES-150553
Riga M, Bibas A, Xenellis J, Korres S (2011) Inner ear disease and benign paroxysmal positional vertigo: a critical review of incidence, clinical characteristics, and management. Int J Otolaryngol 2011:709469. https://doi.org/10.1155/2011/709469
Lechner C, Taylor RL, Todd C, MacDougall H, Yavor R, Halmagyi GM, Welgampola MS (2014) Causes and characteristics of horizontal positional nystagmus. J Neurol 261(5):1009–1017. https://doi.org/10.1007/s00415-013-7223-5
Peng H, Wang L, Song H, Gao B, Yang Y, Lyu F (2023) Clinical characteristics of persistent geotropic horizontal direction-changing positional nystagmus: experience in 189 participants. J Vestib Res. https://doi.org/10.3233/VES-220086. (In press)
Yetiser S, Ince D (2022) Analysis of persistent geotropic and apogeotropic positional nystagmus of the lateral canal benign paroxysmal positional vertigo. J Otol 17(2):90–94. https://doi.org/10.1016/j.joto.2022.01.002
von Brevern M, Radtke A, Clarke AH, Lempert T (2004) Migrainous vertigo presenting as episodic positional vertigo. Neurology 62(3):469–472. https://doi.org/10.1212/01.wnl.0000106949.55346.cd
Kim EK, Pasquesi L, Steenerson KK, Otero-Millan J, Sharon JD (2022) Vestibular test results in patients with horizontal canal benign paroxysmal positional vertigo. Cureus 14(1):e21460. https://doi.org/10.7759/cureus.21460
Celis-Aguilar E, Mayoral-Flores HO, Torrontegui-Zazueta LA, Medina-Cabrera CA, León-Leyva IC, Dehesa-López E (2022) Effectiveness of Brandt Daroff, Semont and Epley maneuvers in the treatment of Benign Paroxysmal Positional Vertigo: a randomized controlled clinical trial. Indian J Otolaryngol Head Neck Surg 74(3):314–321. https://doi.org/10.1007/s12070-021-02516-w
Sinsamutpadung C, Kulthaveesup A (2021) Comparison of outcomes of the Epley and Semont maneuvers in posterior canal BPPV: a randomized controlled trial. Laryngoscope Investig Otolaryngol 6(4):866–871. https://doi.org/10.1002/lio2.619
Strupp M, Goldschagg N, Vinck AS, Bayer O, Vandenbroeck S, Salerni L, Hennig A, Obrist D, Mandalà M (2021) BPPV: comparison of the SémontPLUS with the Sémont Maneuver: a prospective randomized trial. Front Neurol 12:652573. https://doi.org/10.3389/fneur.2021.652573
Mandala M, Salerni L, Nuti D (2019) Benign positional paroxysmal vertigo treatment: a practical update. Curr Treat Opt Neurol 21(12):66. https://doi.org/10.1007/s11940-019-0606-x
Helminski JO (2022) Case report: atypical patterns of nystagmus suggest posterior canal cupulolithiasis and short-arm canalithiasis. Front Neurol 13:982191. https://doi.org/10.3389/fneur.2022.982191
Behr E, Honaker JA (2023) When particle repositioning maneuvers just will not stick: clinical considerations for persistent benign paroxysmal positional vertigo. Am J Audiol. https://doi.org/10.1044/2022_AJA-22-00118. (In press)
Kim HJ, Kim JS, Choi KD, Choi SY, Lee SH, Jung I, Park JH (2023) Effect of self-treatment of recurrent benign paroxysmal positional vertigo: a randomized clinical trial. JAMA Neurol 80(3):244–250. https://doi.org/10.1001/jamaneurol.2022.4944
Giannoni B, Pecci R, Pollastri F, Mininni S, Licci G, Santimone R, Di Giustino F, Mandalà M (2023) Treating benign paroxysmal positional vertigo of the lateral semicircular canal with a shortened forced position. Front Neurol 14:1153491. https://doi.org/10.3389/fneur.2023.1153491
Barreto RG, Yacovino DA, Cherchi M, Teixeira LJ, Nader SN, Leão GF (2023) Universal repositioning maneuver: a new treatment for single canal and multi-canal benign paroxysmal positional vertigo by 3-dimensional model analysis. J Int Adv Otol 19(3):242–247. https://doi.org/10.5152/iao.2023.22921
Bradshaw S, Graco M, Holland A (2023) Barriers and facilitators to guideline-recommended care of benign paroxysmal positional vertigo in the ED: a qualitative study using the theoretical domains framework. Emerg Med J 40(5):335–340. https://doi.org/10.1136/emermed-2022-212585
Edlow JA, Kerber K (2023) Benign paroxysmal positional vertigo: a practical approach for emergency physicians. Acad Emerg Med 30(5):579–588. https://doi.org/10.1111/acem.14558
Neely P, Patel H, Wellings T (2021) Benign paroxysmal positional vertigo in the emergency department: an observational study of an Australian regional hospital’s acute clinical practice. Emerg Med Australas 33(6):1082–1087. https://doi.org/10.1111/1742-6723.13810
Del Risco A, Cherches A, Smith SL, Riska KM (2023) Guideline adherence to benign paroxysmal positional vertigo treatment and management in primary care. Otolaryngol Head Neck Surg. https://doi.org/10.1002/ohn.315. (In press)
Halmagyi GM, McGarvie LA, Strupp M (2020) Nystagmus goggles: how to use them, what you find and what it means. Pract Neurol 20(6):446–450. https://doi.org/10.1136/practneurol-2020-002513
Bech MW, Staffe AT, Hougaard DD (2023) A mechanical rotation chair provides superior diagnostics of benign paroxysmal positional vertigo. Front Neurol 14:1040701. https://doi.org/10.3389/fneur.2023.1040701
Ears ApS (2021) Rotundum. https://ears.dk/rotundum/. Accessed 8 June 2023
Kim JM, Lee SH, Kim HJ, Kim JS (2022) Less talked variants of benign paroxysmal positional vertigo. J Neurol Sci 442:120440. https://doi.org/10.1016/j.jns.2022.120440
Vannucchi P, Pecci R, Giannoni B, Di Giustino F, Santimone R, Mengucci A (2015) Apogeotropic posterior semicircular canal benign paroxysmal positional vertigo: some clinical and therapeutic considerations. Audiol Res 5(1):130. https://doi.org/10.4081/audiores.2015.130
Argaet EC, Young AS, Bradshaw AP, Welgampola MS (2019) Cerebellar arteriovenous malformation presenting with recurrent positional vertigo. J Neurol 266:247–249. https://doi.org/10.1007/s00415-018-9103-5
Lea J, Lechner C, Halmagyi GM, Welgampola MS (2014) Not so benign positional vertigo: paroxysmal downbeat nystagmus from a superior cerebellar peduncle neoplasm. Otol Neurotol 35(6):e204–e205. https://doi.org/10.1097/MAO.0000000000000245
Balatsouras DG, Koukoutsis G, Ganelis P, Economou NC, Moukos A, Aspris A, Katotomichelakis M (2014) Benign paroxysmal positional vertigo secondary to vestibular neuritis. Eur Arch Otorhinolaryngol 271(5):919–924. https://doi.org/10.1007/s00405-013-2484-2
Türk B, Akpinar M, Kaya KS, Korkut AY, Turgut S (2021) Benign paroxysmal positional vertigo: comparison of idiopathic BPPV and BPPV secondary to vestibular neuritis. Ear Nose Throat J 100(7):532–535. https://doi.org/10.1177/0145561319871234
Taylor RL, McGarvie LA, Reid N, Young AS, Halmagyi GM, Welgampola MS (2016) Vestibular neuritis affects both superior and inferior vestibular nerves. Neurology 87(16):1704–1712. https://doi.org/10.1212/WNL.0000000000003223
Kim CH, Choi JM, Jung HV, Park HJ, Shin JE (2014) Sudden sensorineural hearing loss with simultaneous positional vertigo showing persistent geotropic direction-changing positional nystagmus. Otol Neurotol 35(9):1626–1632. https://doi.org/10.1097/MAO.0000000000000457
Pogson JM, Taylor RL, Young AS, McGarvie LA, Flanagan S, Halmagyi GM, Welgampola MS (2016) Vertigo with sudden hearing loss: audio-vestibular characteristics. J Neurol 263(10):2086–2096. https://doi.org/10.1007/s00415-016-8214-0
Rambold H, Heide W, Helmchen C (2004) Horizontal canal benign paroxysmal positioning vertigo with ipsilateral hearing loss. Eur J Neurol 11(1):31–35. https://doi.org/10.1046/j.1351-5101.2003.00705.x
Ahmed S, Heidenreich KD, McHugh JB, Altschuler RA, Carender WJ, Telian SA (2015) Refractory positional vertigo with apogeotropic horizontal nystagmus after labyrinthitis: surgical treatment and identification of dysmorphic ampullae. Otol Neurotol 36(8):1417–1420. https://doi.org/10.1097/MAO.0000000000000820
Pollak L (2009) The importance of repeated clinical examination in patients with suspected benign paroxysmal positional vertigo. Otol Neurotol 30(3):356–358. https://doi.org/10.1097/MAO.0b013e3181967b9c
Celis-Aguilar EM, Medina-Cabrera CA, Torrontegui-Zazueta LA, Núñez-Millán BX, Castro-Bórquez KM, Obeso-Pereda A, García-Valle CG, Ochoa-Miranda CA (2022) Short-term effect of Epley Maneuver as treatment for subjective benign paroxysmal positional vertigo. Indian J Otolaryngol Head Neck Surg 74(Suppl 1):545–549. https://doi.org/10.1007/s12070-020-02320-y
Uz U, Uz D, Akdal G, Çelik O (2019) Efficacy of Epley Maneuver on quality of life of elderly patients with subjective BPPV. J Int Adv Otol 15(3):420–424. https://doi.org/10.5152/iao.2019.6483
Büki B, Mandalà M, Nuti D (2014) Typical and atypical benign paroxysmal positional vertigo: literature review and new theoretical considerations. J Vestib Res 24(5–6):415–423. https://doi.org/10.3233/VES-140535
Martens C, Goplen FK, Nordfalk KF, Aasen T, Nordahl SH (2016) Prevalence and characteristics of positional nystagmus in normal subjects. Otolaryngol Head Neck Surg 154(5):861–867. https://doi.org/10.1177/0194599816629640
Young AS, Rosengren SM, D’Souza M, Bradshaw AP, Welgampola MS (2020) Nystagmus characteristics of healthy controls. J Vestib Res 30(6):345–352. https://doi.org/10.3233/VES-200022
Choi JY, Kim JH, Kim HJ, Glasauer S, Kim JS (2015) Central paroxysmal positional nystagmus: characteristics and possible mechanisms. Neurology 84(22):2238–2246. https://doi.org/10.1212/WNL.0000000000001640
Kim JM, Lee SH, Cho SH, Kang KW, Choi KH, Nam TS, Kim JT, Choi SM, Park MS, Kim BC, Kim MK (2021) Cerebellar infarction presenting with isolated positional vertigo: differentiating factors for benign paroxysmal positional vertigo. Neurol Sci 42(3):1045–1052. https://doi.org/10.1007/s10072-020-04617-w
Lemos J, Strupp M (2022) Central positional nystagmus: an update. J Neurol 269(4):1851–1860. https://doi.org/10.1007/s00415-021-10852-8
Young AS, Nham B, Bradshaw AP, Calic Z, Pogson JM, D’Souza M, Halmagyi GM, Welgampola MS (2021) Clinical, oculographic, and vestibular test characteristics of vestibular migraine. Cephalalgia 41(10):1039–1052. https://doi.org/10.1177/03331024211006042
El-Badry MM, Samy H, Kabel AM, Rafat FM, Sanyelbhaa H (2017) Clinical criteria of positional vertical nystagmus in vestibular migraine. Acta Otolaryngol 137(7):720–722. https://doi.org/10.1080/00016489.2017.1318220
Shemesh AA, Kocoglu K, Akdal G, Ala RT, Halmagyi GM, Zee DS, Otero-Millan J (2022) Modeling the effect of gravity on periodic alternating nystagmus. J Neurol Sci 442:120407. https://doi.org/10.1016/j.jns.2022.120407
Hadjivassiliou M, Manto M, Mitoma H (2022) Rare etiologies in immune-mediated cerebellar ataxias: diagnostic challenges. Brain Sci 12(9):1165. https://doi.org/10.3390/brainsci12091165
Chen JY, Guo ZQ, Wang J, Liu D, Tian E, Guo JQ, Kong WJ, Zhang SL (2023) Vestibular migraine or Meniere’s disease: a diagnostic dilemma. J Neurol 270(4):1955–1968. https://doi.org/10.1007/s00415-022-11532-x
Akdal G, Tanrıverdizade T, Koçoğlu K, Özçelik P, Halmagyi GM, Güneri A, Kirkim G (2023) Menière’s disease with migraine, Menière’s disease without migraine and Vestibular Migraine: clinical differences. J Neurol. https://doi.org/10.1007/s00415-023-11866-0. (In press)
Kim SY, Lee CH, Yoo DM, Kwon MJ, Kim JH, Kim JH, Park B, Lee HJ, Choi HG (2022) Association between Meniere disease and migraine. JAMA Otolaryngol Head Neck Surg 148(5):457–464. https://doi.org/10.1001/jamaoto.2022.0331
Frank M, Abouzari M, Djalilian HR (2023) Meniere’s disease is a manifestation of migraine. Curr Opin Otolaryngol Head Neck Surg. https://doi.org/10.1097/MOO.0000000000000908. (In press)
Lopez-Escamez JA, Carey J, Chung WH, Goebel JA, Magnusson M, Mandalà M, Newman-Toker DE, Strupp M, Suzuki M, Trabalzini F, Bisdorff A, Classification Committee of the Barany Society; Japan Society for Equilibrium Research; European Academy of Otology and Neurotology (EAONO); Equilibrium Committee of the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS); Korean Balance Society (2015) Diagnostic criteria for Menière’s disease. J Vestib Res 25(1):1–7. https://doi.org/10.3233/VES-150549
Yesantharao LV, Donahue M, Smith A, Yan H, Agrawal Y (2022) Virtual audiometric testing using smartphone mobile applications to detect hearing loss. Laryngoscope Investig Otolaryngol 7(6):2002–2010. https://doi.org/10.1002/lio2.928
Lee H, Yi HA, Lee SR, Ahn BH, Park BR (2005) Drop attacks in elderly patients secondary to otologic causes with Meniere’s syndrome or non-Meniere peripheral vestibulopathy. J Neurol Sci 232(1–2):71–76. https://doi.org/10.1016/j.jns.2005.01.012
Perez-Fernandez N, Montes-Jovellar L, Cervera-Paz J, Domenech-Vadillo E (2010) Auditory and vestibular assessment of patients with Ménière’s disease who suffer Tumarkin attacks. Audiol Neurootol 15(6):399–406. https://doi.org/10.1159/000310899
Akdal G, Toydemir HE, Tanrıverdizade T, Halmagyi GM (2017) Room tilt illusion: a symptom of both peripheral and central vestibular disorders. Acta Neurol Belg 117(1):363–365. https://doi.org/10.1007/s13760-016-0628-z
Arntzen K, Alstadhaug KB (2020) Room tilt illusion and subclavian steal—a case report. BMC Neurol 20(1):369. https://doi.org/10.1186/s12883-020-01947-2
Fattal D, Wattiez AS, St Louis E, Gonzalez-Otarula K, Sainju R (2021) Room tilt illusion in epilepsy. Epileptic Disord 23(6):901–905. https://doi.org/10.1684/epd.2021.1332
Kwon H, Kwon E, Kim HJ, Choi JY, Kim JS (2022) Vestibular syncope: clinical characteristics and mechanism. Ann Clin Transl Neurol 9(10):1616–1625. https://doi.org/10.1002/acn3.51661
McGarvie LA, Curthoys IS, MacDougall HG, Halmagyi GM (2015) What does the dissociation between the results of video head impulse versus caloric testing reveal about the vestibular dysfunction in Ménière’s disease? Acta Otolaryngol 135(9):859–865. https://doi.org/10.3109/00016489.2015.1015606
Hannigan IP, Rosengren SM, Young AS, Bradshaw AP, Calic Z, Kwok B, Alraddy B, Gibson WPR, Kong J, Flanagan S, Halmagyi GM, Watson SRD, Welgampola MS (2022) A portrait of Menière’s disease using contemporary hearing and balance tests. Otol Neurotol 43(4):e489–e496. https://doi.org/10.1097/MAO.0000000000003479
Hannigan IP, Welgampola MS, Watson SRD (2021) Dissociation of caloric and head impulse tests: a marker of Meniere’s disease. J Neurol 268(2):431–439. https://doi.org/10.1007/s00415-019-09431-9
Kim HS, Oh EH, Kim JY, Choi SY, Choi KD, Choi JH (2022) Discordant vestibulo-ocular reflex function according to the frequency and mode of stimulation. J Neurol 269(9):4742–4752. https://doi.org/10.1007/s00415-022-11105-y
Nham B, Welgampola MS, Halmagyi GM (2020) Contralesional subjective visual horizontal predicts endolymphatic hydrops. Acta Otolaryngol 140(10):833–837. https://doi.org/10.1080/00016489.2020.1774650
Young AS, Nham B, Bradshaw AP, Calic Z, Pogson JM, Gibson WP, Halmagyi GM, Welgampola MS (2022) Clinical, oculographic and vestibular test characteristics of Ménière’s disease. J Neurol 269(4):1927–1944. https://doi.org/10.1007/s00415-021-10699-z
Lee SU, Kee HJ, Sheen SS, Choi BY, Koo JW, Kim JS (2015) Head-shaking and vibration-induced nystagmus during and between the attacks of unilateral Ménière’s disease. Otol Neurotol 36(5):865–872. https://doi.org/10.1097/MAO.0000000000000743
Yacovino DA, Finlay JB (2016) Intra-attack vestibuloocular reflex changes in Ménière’s disease. Case Rep Otolaryngol 2016:2427983. https://doi.org/10.1155/2016/2427983
Yacovino DA, Hain TC, Musazzi M (2017) Fluctuating vestibulo-ocular reflex in Ménière’s disease. Otol Neurotol 38(2):244–247. https://doi.org/10.1097/MAO.0000000000001298
Yacovino DA, Schubert MC, Zanotti E (2020) Evidence of large vestibulo-ocular reflex reduction in patients with Menière attacks. Otol Neurotol 41(9):e1133–e1139. https://doi.org/10.1097/MAO.0000000000002746
Curthoys IS, Manzari L, Rey-Martinez J, Dlugaiczyk J, Burgess AM (2021) Enhanced eye velocity in head impulse testing—a possible indicator of endolymphatic hydrops. Front Surg 8:666390. https://doi.org/10.3389/fsurg.2021.666390
Aw ST, Aw GE, Todd MJ, Halmagyi GM (2013) Enhanced vestibulo-ocular reflex to electrical vestibular stimulation in Meniere's disease. J Assoc Res Otolaryngol 14(1):49–59. https://doi.org/10.1007/s10162-012-0362-z
Lucas JC, Hong RS (2023) Recent surgical advances and continued controversies in medically refractory Meniere’s disease. Curr Opin Otolaryngol Head Neck Surg. https://doi.org/10.1097/MOO.0000000000000907. (In press)
Faizal B, Rajan A (2022) Low dose intratympanic gentamicin in Ménière’s disease. Indian J Otolaryngol Head Neck Surg 74(Suppl 1):320–325. https://doi.org/10.1007/s12070-020-02104-4
Lacour M, Bernard-Demanze L (2015) Interaction between vestibular compensation mechanisms and vestibular rehabilitation therapy: 10 recommendations for optimal functional recovery. Front Neurol 5:285. https://doi.org/10.3389/fneur.2014.00285
David EA, Shahnaz N (2023) Dynamic posturography after computerized vestibular retraining for stable unilateral vestibular deficits. Acta Otolaryngol 143(5):396–401. https://doi.org/10.1080/00016489.2023.2208615
Lilios A, Chimona T, Papadakis C, Chatziioanou I, Nikitas C, Skoulakis C (2023) Different vestibular rehabilitation modalities in unilateral vestibular hypofunction: a prospective study. Otol Neurotol 44(4):e246–e255. https://doi.org/10.1097/MAO.0000000000003836
Scheltinga A, Honegger F, Timmermans DP, Allum JH (2016) The effect of age on improvements in vestibulo-ocular reflexes and balance control after acute unilateral peripheral vestibular loss. Front Neurol 7:18. https://doi.org/10.3389/fneur.2016.00018
Hilton A, McClelland A, McCallum R, Kontorinis G (2022) Duration of symptom control following intratympanic dexamethasone injections in Meniere’s disease. Eur Arch Otorhinolaryngol 279(11):5191–5198. https://doi.org/10.1007/s00405-022-07368-w
Shim T, Strum DP, Mudry A, Monfared A (2020) Hold the salt: history of salt restriction as a first-line therapy for Menière’s disease. Otol Neurotol 41(6):855–859. https://doi.org/10.1097/MAO.0000000000002635
Szott FA, Westhofen M, Hackenberg S (2023) Is endolymphatic sac surgery an efficient treatment of Menière’s disease patients? A systematic literature search and meta-analysis. Eur Arch Otorhinolaryngol 280(3):1119–1128. https://doi.org/10.1007/s00405-022-07580-8 (Erratum in: Eur Arch Otorhinolaryngol 2023:1129. https://doi.org/10.1007/s00405-022-07717-9)
Conway RM, Babu SC, Mallany P, Weymon A, Wilkerson BJ (2023) Endolymphatic sac decompression effect on secondary symptoms of Meniere’s disease. Am J Otolaryngol 44(2):103777. https://doi.org/10.1016/j.amjoto.2022.103777
Van Esch B, van der Zaag-Loonen H, Bruintjes T, van Benthem PP (2022) Betahistine in Ménière’s disease or syndrome: a systematic review. Audiol Neurootol 27(1):1–33. https://doi.org/10.1159/000515821
Van de Heyning P, Betka J, Chovanec M, Devèze A, Giannuzzi AL, Krempaská S, Przewoźny T, Scheich M, Strupp M, Van Rompaey V, Meyer T (2023) Efficacy and safety of intranasal betahistine in the treatment of surgery-induced acute vestibular syndrome: a double-blind, randomized, placebo-controlled phase 2 study. Otol Neurotol 44(5):493–501. https://doi.org/10.1097/MAO.0000000000003856
Scholtz AW, Waldfahrer F, Hampel R, Weisshaar G (2022) Efficacy and safety of a fixed-dose combination of cinnarizine 20 mg and dimenhydrinate 40 mg in the treatment of patients with vestibular vertigo: an individual patient data meta-analysis of randomised, double-blind, controlled clinical trials. Clin Drug Investig 42(9):705–720. https://doi.org/10.1007/s40261-022-01184-0
Akdal G, Ozge A, Ergör G (2013) The prevalence of vestibular symptoms in migraine or tension-type headache. J Vestib Res 23(2):101–106. https://doi.org/10.3233/VES-130477
Akdal G, Baykan B, Ertas M, Zarifoglu M, Karli N, Saip S, Siva A (2015) Population-based study of vestibular symptoms in migraineurs. Acta Otolaryngol 135(5):435–439. https://doi.org/10.3109/00016489.2014.969382
Akdal G, Özge A, Ergör G (2015) Vestibular symptoms are more frequent in migraine than in tension type headache patients. J Neurol Sci 357(1–2):295–296. https://doi.org/10.1016/j.jns.2015.06.059
Cho SJ, Kim BK, Kim BS, Kim JM, Kim SK, Moon HS, Song TJ, Cha MJ, Park KY, Sohn JH (2016) Vestibular migraine in multicenter neurology clinics according to the appendix criteria in the third beta edition of the International Classification of Headache Disorders. Cephalalgia 36(5):454–462. https://doi.org/10.1177/0333102415597890
Dieterich M, Obermann M, Celebisoy N (2016) Vestibular migraine: the most frequent entity of episodic vertigo. J Neurol 263(Suppl 1):82–89. https://doi.org/10.1007/s00415-015-7905-2
Çelebisoy N, Ak AK, Ataç C, Özdemir HN, Gökçay F, Durmaz GS, Kartı DT, Toydemir HE, Yayla V, Işıkay İÇ, Erkent İ, Sarıtaş AŞ, Özçelik P, Akdal G, Bıçakcı Ş, Göksu EO, Uyaroğlu FG (2023) Comparison of clinical features in patients with vestibular migraine and migraine. J Neurol 270(7):3567–3573. https://doi.org/10.1007/s00415-023-11677-3
Neuhauser H, Leopold M, von Brevern M, Arnold G, Lempert T (2001) The interrelations of migraine, vertigo, and migrainous vertigo. Neurology 56(4):436–441. https://doi.org/10.1212/wnl.56.4.436
Akdal G, Özçelik P, Özge A (2020) Vestibular migraine: considered from both the vestibular and the migraine point of view. Neurol Sci Neurophysiol 37(2):41–49. https://doi.org/10.4103/NSN.NSN_72_20
Lempert T, Olesen J, Furman J, Waterston J, Seemungal B, Carey J, Bisdorff A, Versino M, Evers S, Kheradmand A, Newman-Toker D (2022) Vestibular migraine: diagnostic criteria (Update). J Vestib Res 32(1):1–6. https://doi.org/10.3233/VES-201644
Çelebisoy N, Kısabay Ak A, Özdemir HN, Gökçay F, Durmaz GS, Kartı DT, Toydemir HE, Yayla V, Çolpak Işıkay Aİ, Erkent İ, Özçelik P, Akdal G, Ataç C, Bıçakcı Ş, Göksu EO, Uyaroğlu FG (2022) Vestibular migraine, demographic and clinical features of 415 patients: a multicenter study. Clin Neurol Neurosurg 215:107201. https://doi.org/10.1016/j.clineuro.2022.107201
Eggers SD, Neff BA, Shepard NT, Staab JP (2014) Comorbidities in vestibular migraine. J Vestib Res 24(5–6):387–395. https://doi.org/10.3233/VES-140525
Sharon JD, Hullar TE (2014) Motion sensitivity and caloric responsiveness in vestibular migraine and Meniere’s disease. Laryngoscope 124(4):969–973. https://doi.org/10.1002/lary.24285
Golding JF, Patel M (2017) Meniere’s, migraine, and motion sickness. Acta Otolaryngol 137(5):495–502. https://doi.org/10.1080/00016489.2016.1255775
Cha YH, Cui Y (2013) Rocking dizziness and headache: a two-way street. Cephalalgia 33(14):1160–1169. https://doi.org/10.1177/0333102413487999
Beh SC, Chiang HS, Sanderson C (2021) The interconnections of Mal de Débarquement syndrome and vestibular migraine. Laryngoscope 131(5):E1653–E1661. https://doi.org/10.1002/lary.29214
Akdal G, Balci BD, Angin S, Oztürk V, Halmagyi GM (2012) A longitudinal study of balance in migraineurs. Acta Otolaryngol 132(1):27–32. https://doi.org/10.3109/00016489.2011.616532
Balci B, Akdal G (2022) Outcome of vestibular rehabilitation in vestibular migraine. J Neurol 269(12):6246–6253. https://doi.org/10.1007/s00415-022-11250-4
Özçelik P, Koçoğlu K, Öztürk V, Keskinoğlu P, Akdal G (2022) Characteristic differences between vestibular migraine and migraine only patients. J Neurol 269(1):336–341. https://doi.org/10.1007/s00415-021-10636-0
Kırkım G, Mutlu B, Olgun Y, Tanriverdizade T, Keskinoğlu P, Güneri EA, Akdal G (2017) Comparison of audiological findings in patients with vestibular migraine and migraine. Turk Arch Otorhinolaryngol 55(4):158–161. https://doi.org/10.5152/tao.2017.2609
van de Berg R, Widdershoven J, Bisdorff A, Evers S, Wiener-Wacher S, Cushing SL, Mack KJ, Kim JS, Jahn K, Strupp M, Lempert T (2021) Vestibular migraine of childhood and recurrent vertigo of childhood: diagnostic criteria consensus document of the Committee for the Classification of Vestibular Disorders of the Bárány Society and the International Headache Society. J Vestib Res 31(1):1–9. https://doi.org/10.3233/VES-200003
Cokyaman T, Cetin H (2022) Pediatric vestibular migraine: diagnosis according to ICHD-3 criteria and the effectiveness of short-term CH prophylaxis. Eur J Paediatr Neurol 39:19–24. https://doi.org/10.1016/j.ejpn.2022.05.002
Wang A, Zhou G, Brodsky JR (2023) Characteristics of benign paroxysmal positional vertigo in young children. Laryngoscope 133(3):694–699. https://doi.org/10.1002/lary.30172
Gedik-Soyuyuce O, Gence-Gumus Z, Ozdilek A, Ada M, Korkut N (2021) Vestibular disorders in children: a retrospective analysis of vestibular function test findings. Int J Pediatr Otorhinolaryngol 146:110751. https://doi.org/10.1016/j.ijporl.2021.110751
Lahiji MR, Akbarpour M, Soleimani R, Asli RH, Leyli EK, Saberi A, Akbari M, Ramezani H, Nemati S (2022) Prevalence of anxiety and depression in Meniere’s disease; a comparative analytical study. Am J Otolaryngol 43(5):103565. https://doi.org/10.1016/j.amjoto.2022.103565
Balcı B, Akdal G (2020) Imbalance, motion sensitivity, anxiety and handicap in vestibular migraine and migraine only patients. Auris Nasus Larynx 47(5):747–751. https://doi.org/10.1016/j.anl.2020.02.015
Kutay Ö, Akdal G, Keskinoğlu P, Balcı BD, Alkın T (2017) Vestibular migraine patients are more anxious than migraine patients without vestibular symptoms. J Neurol 264(Suppl 1):37–41. https://doi.org/10.1007/s00415-017-8439-6
Smitherman TA, Kolivas ED, Bailey JR (2013) Panic disorder and migraine: comorbidity, mechanisms, and clinical implications. Headache 53(1):23–45. https://doi.org/10.1111/head.12004
Penkava J, Bardins S, Brandt T, Wuehr M, Huppert D (2020) Spontaneous visual exploration during locomotion in patients with phobic postural vertigo. J Neurol 267(Suppl 1):223–230. https://doi.org/10.1007/s00415-020-10151-8
Obermann M, Gebauer A, Arweiler-Harbeck D, Lang S, Seilheimer B, Kleinschnitz C, Diener HC, Holle D, Naegel S (2023) Cognitive deficits in patients with peripheral vestibular dysfunction. Eur J Neurol. https://doi.org/10.1111/ene.15907. (Online ahead of print)
Dornhoffer JR, Liu YF, Zhao EE, Rizk HG (2021) Does cognitive dysfunction correlate with dizziness severity in Meniére’s disease patients. Otol Neurotol 42(3):e323–e331. https://doi.org/10.1097/MAO.0000000000002958
Chari DA, Liu YH, Chung JJ, Rauch SD (2021) Subjective cognitive symptoms and Dizziness Handicap Inventory (DHI) performance in patients with vestibular migraine and Menière’s disease. Otol Neurotol 42(6):883–889. https://doi.org/10.1097/MAO.0000000000003081
Demirhan MA, Celebisoy N (2023) Cognitive functions in episodic vestibular disorders: Meniere’s disease and vestibular migraine. J Vestib Res 33(1):63–70. https://doi.org/10.3233/VES-220025
Eraslan Boz H, Kırkım G, Koçoğlu K, Çakır Çetin A, Akkoyun M, Güneri EA, Akdal G (2023) Cognitive function in Meniere’s disease. Psychol Health Med 28(4):1076–1086. https://doi.org/10.1080/13548506.2022.2144637
Xie D, Welgampola MS, Miller LA, Young AS, D’Souza M, Breen N, Rosengren SM (2022) Subjective cognitive dysfunction in patients with dizziness and vertigo. Audiol Neurootol 27(2):122–132. https://doi.org/10.1159/000518188
Patel EJ, Hum M, Gardi A, Steenerson KK, Rizk HG, Sharon JD (2023) VM-PATHI Correlates With Cognitive Function Improvement After Successful Treatment in Patients With Vestibular Migraine. Otol Neurotol. (In press.) https://doi.org/10.1097/MAO.0000000000003976
von Brevern M, Zeise D, Neuhauser H, Clarke AH, Lempert T (2005) Acute migrainous vertigo: clinical and oculographic findings. Brain 128:365–374. https://doi.org/10.1093/brain/awh351
Oh SY, Seo MW, Kim YH, Choi KD, Kim DS, Shin BS (2009) Gaze-evoked and rebound nystagmus in a case of migrainous vertigo. J Neuroophthalmol 29(1):26–28. https://doi.org/10.1097/WNO.0b013e318198c910
Yu J, Yu Q, Guan B, Lu Y, Chen C, Yu S (2020) Pseudo-benign paroxysmal positional vertigo: a retrospective study and case report. Front Neurol 11:187. https://doi.org/10.3389/fneur.2020.00187
Neff BA, Staab JP, Eggers SD, Carlson ML, Schmitt WR, Van Abel KM, Worthington DK, Beatty CW, Driscoll CL, Shepard NT (2012) Auditory and vestibular symptoms and chronic subjective dizziness in patients with Ménière’s disease, vestibular migraine, and Ménière’s disease with concomitant vestibular migraine. Otol Neurotol 33(7):1235–1244. https://doi.org/10.1097/MAO.0b013e31825d644a
Lopez-Escamez JA, Dlugaiczyk J, Jacobs J, Lempert T, Teggi R, von Brevern M, Bisdorff A (2014) Accompanying symptoms overlap during attacks in Menière’s disease and vestibular migraine. Front Neurol 5:265. https://doi.org/10.3389/fneur.2014.00265
Murofushi T, Tsubota M, Kitao K, Yoshimura E (2018) Simultaneous presentation of definite vestibular migraine and definite Ménière’s disease: overlapping syndrome of two diseases. Front Neurol 9:749. https://doi.org/10.3389/fneur.2018.00749
Pollak L, Pollak E (2014) Headache during a cluster of benign paroxysmal positional vertigo attacks. Ann Otol Rhinol Laryngol 123(12):875–880. https://doi.org/10.1177/0003489414539921
Chu CH, Liu CJ, Lin LY, Chen TJ, Wang SJ (2015) Migraine is associated with an increased risk for benign paroxysmal positional vertigo: a nationwide population-based study. J Headache Pain 16:62. https://doi.org/10.1186/s10194-015-0547-z
Bruss D, Abouzari M, Sarna B, Goshtasbi K, Lee A, Birkenbeuel J, Djalilian HR (2021) Migraine features in patients with recurrent benign paroxysmal positional vertigo. Otol Neurotol 42(3):461–465. https://doi.org/10.1097/MAO.0000000000002976
Sharpe L, Dudeney J, Williams ACC, Nicholas M, McPhee I, Baillie A, Welgampola M, McGuire B (2019) Psychological therapies for the prevention of migraine in adults. Cochrane Database Syst Rev 7(7):CD012295. https://doi.org/10.1002/14651858.CD012295.pub2
Webster K, Dor A, Galbraith K, Kassem LH, Harrington-Benton N, Judd O, Kaski D, Maarsingh O, MacKeith S, Ray J, Van Vugt V, Burton M (2023) Pharmacological interventions for prophylaxis of vestibular migraine. Cochrane Database Syst Rev 4:CD015187. https://doi.org/10.1002/14651858.CD015187.pub2
Webster KE, Dor A, Galbraith K, Haj Kassem L, Harrington-Benton NA, Judd O, Kaski D, Maarsingh OR, MacKeith S, Ray J, Van Vugt VA, Burton MJ (2023) Pharmacological interventions for acute attacks of vestibular migraine. Cochrane Database Syst Rev 4(4):CD015322. https://doi.org/10.1002/14651858.CD015322.pub2
Webster KE, Dor A, Galbraith K, Haj Kassem L, Harrington-Benton NA, Judd O, Kaski D, Maarsingh OR, MacKeith S, Ray J, Van Vugt VA, Burton MJ (2023) Non-pharmacological interventions for prophylaxis of vestibular migraine. Cochrane Database Syst Rev 4(4):CD015321. https://doi.org/10.1002/14651858.CD015321.pub2
Kısabay Ak A, Çelebisoy N, Özdemir HN, Gökçay F, Saruhan Durmaz G, Top Kartı D, Ertaşoğlu Toydemir H, Yayla V, Çolpak Işıkay Aİ, Erkent İ, Özçelik P, Akdal G, Ataç C, Bıçakcı Ş, Ozaydın-Göksu E, Güleç Uyaroğlu F (2022) Factors determining the response to treatment in patients with vestibular migraine. Neurol Res 44(9):847–854. https://doi.org/10.1080/01616412.2022.2056341
Aydin I, Gökçay F, Karapolat H, Eraslan S, Bilgen C, Kirazli T, Tanıgör G, Köse T, Çelebisoy N (2020) Effects of vestibular rehabilitation and pharmacological therapy in patients with vestibular migraine. Neurol Sci Neurophysiol 37(3):110–117. https://doi.org/10.4103/NSN.NSN_41_20
Smyth D, Britton Z, Murdin L, Arshad Q, Kaski D (2022) Vestibular migraine treatment: a comprehensive practical review. Brain 145(11):3741–3754. https://doi.org/10.1093/brain/awac264
Young AS, Lechner C, Bradshaw AP, MacDougall HG, Black DA, Halmagyi GM, Welgampola MS (2019) Capturing acute vertigo: a vestibular event monitor. Neurology 92(24):e2743–e2753. https://doi.org/10.1212/WNL.0000000000007644
Goldberg JM, Fernandez C (1971) Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. I. Resting discharge and response to constant angular accelerations. J Neurophysiol 34(4):635–660. https://doi.org/10.1152/jn.1971.34.4.635
Shinoda Y, Yoshida K (1974) Dynamic characteristics of responses to horizontal head angular acceleration in vestibuloocular pathway in the cat. J Neurophysiol 37(4):653–673. https://doi.org/10.1152/jn.1974.37.4.653
Aw ST, Fetter M, Cremer PD, Karlberg M, Halmagyi GM (2001) Individual semicircular canal function in superior and inferior vestibular neuritis. Neurology 57(5):768–774. https://doi.org/10.1212/wnl.57.5.768
MacDougall HG, McGarvie LA, Halmagyi GM, Curthoys IS, Weber KP (2013) The video Head Impulse Test (vHIT) detects vertical semicircular canal dysfunction. PLoS One 8(4):e61488. https://doi.org/10.1371/journal.pone.0061488
MacDougall HG, Weber KP, McGarvie LA, Halmagyi GM, Curthoys IS (2009) The video head impulse test: diagnostic accuracy in peripheral vestibulopathy. Neurology 73(14):1134–1141. https://doi.org/10.1212/WNL.0b013e3181bacf85
Halmagyi GM, Curthoys IS (1988) A clinical sign of canal paresis. Arch Neurol 45(7):737–739. https://doi.org/10.1001/archneur.1988.00520310043015
Halmagyi GM, Curthoys IS (2018) The video head impulse test in clinical practice. Neurol Sci Neurophysiol 35:1–5. https://doi.org/10.5152/NSN.2018.0001
Yip CW, Glaser M, Frenzel C, Bayer O, Strupp M (2016) Comparison of the bedside head-impulse test with the video head-impulse test in a clinical practice setting: a prospective study of 500 outpatients. Front Neurol 7:58. https://doi.org/10.3389/fneur.2016.00058
George M, Kolethekkat AA, Yoan P, Maire R (2023) Video Head impulse test: a comparison and analysis of three recording systems. Indian J Otolaryngol Head Neck Surg 75(1):60–66. https://doi.org/10.1007/s12070-022-03170-6
Curthoys IS, MacDougall HG, McGarvie LA, Weber KP, Szmulewicz D, Manzari L, Burgess AM, Halmagyi GM (2014) The video head impulse test (vHIT). In: Jacobson GP, Shepard NT (eds) Balance function assessment and management, chapter 16, 2nd edn. Plural Publishing, San Diego, pp 391–430
Koohi N, Mendis S, Lennox A, Whelan D, Kaski D (2022) Video head impulse testing: pitfalls in neurological patients. J Neurol Sci 442:120417. https://doi.org/10.1016/j.jns.2022.120417
Korda A, Sauter TC, Caversaccio MD, Mantokoudis G (2021) Quantifying a learning curve for video head impulse test: pitfalls and pearls. Front Neurol 11:615651. https://doi.org/10.3389/fneur.2020.615651
McCaslin DL, Rivas A, Jacobson GP, Bennett ML (2015) The dissociation of video head impulse test (vHIT) and bithermal caloric test results provide topological localization of vestibular system impairment in patients with “definite” Ménière’s disease. Am J Audiol 24(1):1–10. https://doi.org/10.1044/2014_AJA-14-0040
Herdman SJ, Hall CD, Maloney B, Knight S, Ebert M, Lowe J (2015) Variables associated with outcome in patients with bilateral vestibular hypofunction: preliminary study. J Vestib Res 25(3–4):185–194. https://doi.org/10.3233/VES-150556
Curthoys IS, McGarvie LA, MacDougall HG, Burgess AM, Halmagyi GM, Rey-Martinez J, Dlugaiczyk J (2023) A review of the geometrical basis and the principles underlying the use and interpretation of the video head impulse test (vHIT) in clinical vestibular testing. Front Neurol 14:1147253. https://doi.org/10.3389/fneur.2023.1147253
Nguyen J, Berger J, Curthoys I, Held V, Zaubitzer L, Hülse R, Rotter N, Schell A (2021) Vestibular testing in children—the suppression head impulse (SHIMP) test. Int J Pediatr Otorhinolaryngol 151:110921. https://doi.org/10.1016/j.ijporl.2021.110921
Rodríguez-Villalba R, Caballero-Borrego M (2023) Normative values for the video head impulse test in children without otoneurologic symptoms and their evolution across childhood by gender. Eur Arch Otorhinolaryngol. https://doi.org/10.1007/s00405-023-07900-6. (In press)
Newman-Toker DE, Curthoys IS, Halmagyi GM (2015) Diagnosing stroke in acute vertigo: the HINTS family of eye movement tests and the future of the “Eye ECG.” Semin Neurol 35(5):506–521. https://doi.org/10.1055/s-0035-1564298
Edlow JA, Carpenter C, Akhter M, Khoujah D, Marcolini E, Meurer WJ, Morrill D, Naples JG, Ohle R, Omron R, Sharif S, Siket M, Upadhye S, Silva LOJE, Sundberg E, Tartt K, Vanni S, Newman-Toker DE, Bellolio F (2023) Guidelines for reasonable and appropriate care in the emergency department 3 (GRACE-3): acute dizziness and vertigo in the emergency department. Acad Emerg Med 30(5):442–486. https://doi.org/10.1111/acem.14728
Gerlier C, Fels A, Vitaux H, Mousset C, Perugini A, Chatellier G, Ganansia O (2023) Effectiveness and reliability of the four-step STANDING algorithm performed by interns and senior emergency physicians for predicting central causes of vertigo. Acad Emerg Med 30(5):487–500. https://doi.org/10.1111/acem.14659
Calic Z, Nham B, Bradshaw AP, Young AS, Bhaskar S, D’Souza M, Anderson CS, Cappelen-Smith C, Cordato D, Welgampola MS (2020) Separating posterior-circulation stroke from vestibular neuritis with quantitative vestibular testing. Clin Neurophysiol 131(8):2047–2055. https://doi.org/10.1016/j.clinph.2020.04.173
Nham B, Wang C, Reid N, Calic Z, Kwok BYC, Black DA, Bradshaw A, Halmagyi G, Welgampola MS (2023) Modern vestibular tests can accurately separate stroke and vestibular neuritis. J Neurol 270(4):2031–2041. https://doi.org/10.1007/s00415-022-11473-5
Nham B, Akdal G, Young AS, Özçelik P, Tanrıverdizade T, Ala RT, Bradshaw AP, Wang C, Men S, Giarola BF, Black DA, Thompson EO, Halmagyi GM, Welgampola MS (2023) Capturing nystagmus in the emergency room: posterior circulation stroke versus acute vestibular neuritis. J Neurol 270(2):632–641. https://doi.org/10.1007/s00415-022-11202-y
Thomas JO, Sharobeam A, Venkat A, Blair C, Ozalp N, Calic Z, Wyllie P, Middleton PM, Welgampola M, Cordato D, Cappelen-Smith C (2022) Video head impulse testing to differentiate vestibular neuritis from posterior circulation stroke in the emergency department: a prospective observational study. BMJ Neurol Open 4(1):e000284. https://doi.org/10.1136/bmjno-2022-000284
Nham B, Reid N, Bein K, Bradshaw AP, McGarvie LA, Argaet EC, Young AS, Watson SR, Halmagyi GM, Black DA, Welgampola MS (2022) Capturing vertigo in the emergency room: three tools to double the rate of diagnosis. J Neurol 269(1):294–306. https://doi.org/10.1007/s00415-021-10627-1
Strupp M, Bisdorff A, Furman J, Hornibrook J, Jahn K, Maire R, Newman-Toker D, Magnusson M (2022) Acute unilateral vestibulopathy/vestibular neuritis: diagnostic criteria. J Vestib Res 32(5):389–406. https://doi.org/10.3233/VES-220201
Mantokoudis G, Tehrani AS, Wozniak A, Eibenberger K, Kattah JC, Guede CI, Zee DS, Newman-Toker DE (2015) VOR gain by head impulse video-oculography differentiates acute vestibular neuritis from stroke. Otol Neurotol 36(3):457–465. https://doi.org/10.1097/MAO.0000000000000638
Hannigan IP, Nham B, Wang C, Rosengren SM, Kwok BYC, McGarvie LA, Reid NM, Curthoys IS, Halmágyi GM, Welgampola MS (2023) The relationship between the subjective visual horizontal and ocular vestibular evoked myogenic potentials in acute vestibular neuritis. Otol Neurotol 44(6):e419–e427. https://doi.org/10.1097/MAO.0000000000003909
Argaet EC, Kwok BYC, Bradley J, Young AS, Nham B, Calic Z, Taylor RL, Pogson JM, Reid N, Kong JHK, Flanagan S, Halmagyi GM, Rosengren SM, Welgampola MS (2023) Subjective visual horizontal correlates better with ocular than with cervical vestibular evoked myogenic potentials. Clin Neurophysiol 152:1–10. https://doi.org/10.1016/j.clinph.2023.04.011
Rosengren SM, Colebatch JG, Young AS, Govender S, Welgampola MS (2019) Vestibular evoked myogenic potentials in practice: Methods, pitfalls and clinical applications. Clin Neurophysiol Pract 4:47–68. https://doi.org/10.1016/j.cnp.2019.01.005
Taylor RL, Welgampola MS, Nham B, Rosengren SM (2020) Vestibular-evoked myogenic potential testing in vestibular localization and diagnosis. Semin Neurol 40(1):18–32. https://doi.org/10.1055/s-0039-3402068
Chen L, Todd M, Halmagyi GM, Aw S (2014) Head impulse gain and saccade analysis in pontine-cerebellar stroke and vestibular neuritis. Neurology 83(17):1513–1522. https://doi.org/10.1212/WNL.0000000000000906
Choi JY, Kim HJ, Kim JS (2018) Recent advances in head impulse test findings in central vestibular disorders. Neurology 90(13):602–612. https://doi.org/10.1212/WNL.0000000000005206
Kim SH, Lee SU, Cho BH, Cho KH, Yu S, Kim BJ, Kim JS (2023) Analyses of head-impulse tests in patients with posterior circulation stroke and vestibular neuritis. Neurology 100(23):e2374–e2385. https://doi.org/10.1212/WNL.0000000000207299
Carmona S, Martínez C, Zalazar G, Koohi N, Kaski D (2023) Acute truncal ataxia without nystagmus in patients with acute vertigo. Eur J Neurol 30(6):1785–1790. https://doi.org/10.1111/ene.15729
Calic Z, Cappelen-Smith C, Anderson CS, Xuan W, Cordato DJ (2016) Cerebellar infarction and factors associated with delayed presentation and misdiagnosis. Cerebrovasc Dis 42(5–6):476–484. https://doi.org/10.1159/000448899
Lucia K, Reitz S, Hattingen E, Steinmetz H, Seifert V, Czabanka M (2023) Predictors of clinical outcomes in space-occupying cerebellar infarction undergoing suboccipital decompressive craniectomy. Front Neurol 14:1165258. https://doi.org/10.3389/fneur.2023.1165258
David AM, Jaleel A, Joy Mathew CM (2023) Misdiagnosis of cerebellar infarcts and its outcome. Cureus 15(2):e35362. https://doi.org/10.7759/cureus.35362
Saber Tehrani AS, Kattah JC, Mantokoudis G, Pula JH, Nair D, Blitz A, Ying S, Hanley DF, Zee DS, Newman-Toker DE (2014) Small strokes causing severe vertigo: frequency of false-negative MRIs and nonlacunar mechanisms. Neurology 83(2):169–173. https://doi.org/10.1212/WNL.0000000000000573
Smith P (2013) In a spin: acute vestibular neuritis. Pract Neurol 13(5):326–327. https://doi.org/10.1136/practneurol-2013-000596
Lacour M, Helmchen C, Vidal PP (2016) Vestibular compensation: the neuro-otologist’s best friend. J Neurol 263(Suppl 1):S54–S64. https://doi.org/10.1007/s00415-015-7903-4
Adamec I, Krbot Skorić M, Ozretić D, Habek M (2014) Predictors of development of chronic vestibular insufficiency after vestibular neuritis. J Neurol Sci 347(1–2):224–228. https://doi.org/10.1016/j.jns.2014.10.001
Cleworth TW, Kessler P, Honegger F, Carpenter MG, Allum JHJ (2022) Vestibulo-ocular reflex gain improvements at peak head acceleration and velocity following onset of unilateral vestibular neuritis: Insights into neural compensation mechanisms. J Vestib Res 32(6):517–527. https://doi.org/10.3233/VES-210153
Cleworth TW, Allum JHJ, Luu MJ, Lea J, Westerberg BW, Carpenter MG (2020) The effect of unilateral vestibular loss on standing balance during postural threat. Otol Neurotol 41(7):e945–e951. https://doi.org/10.1097/MAO.0000000000002485
Halmágyi GM, Curthoys IS (2021) Vestibular contributions to the Romberg test: testing semicircular canal and otolith function. Eur J Neurol 28(9):3211–3219. https://doi.org/10.1111/ene.14942
Rambold HA (2015) Prediction of short-term outcome in acute superior vestibular nerve failure: three-dimensional video-head-impulse test and caloric irrigation. Int J Otolaryngol 2015:639024. https://doi.org/10.1155/2015/639024
Allum JH, Cleworth T, Honegger F (2016) Recovery of vestibulo-ocular reflex symmetry after an acute unilateral peripheral vestibular deficit: time course and correlation with canal paresis. Otol Neurotol 37(6):772–780. https://doi.org/10.1097/MAO.0000000000001054
McGarvie LA, MacDougall HG, Curthoys IS, Halmagyi GM (2020) Spontaneous recovery of the vestibulo-ocular reflex after vestibular neuritis; long-term monitoring with the video head impulse test in a single patient. Front Neurol 11:732. https://doi.org/10.3389/fneur.2020.00732
Oliveira JESL, Khoujah D, Naples JG, Edlow JA, Gerberi DJ, Carpenter CR, Bellolio F (2023) Corticosteroids for patients with vestibular neuritis: an evidence synthesis for guidelines for reasonable and appropriate care in the emergency department. Acad Emerg Med 30(5):531–540. https://doi.org/10.1111/acem.14583
Bogdanova A, Dlugaiczyk J, Heckmann JG, Schwab S (2022) Corticosteroids in patients with vestibular neuritis: an updated meta-analysis. Acta Neurol Scand 146(5):429–439. https://doi.org/10.1111/ane.13676
Halmagyi GM, Weber KP, Curthoys IS (2010) Vestibular function after acute vestibular neuritis. Restor Neurol Neurosci 28(1):37–46. https://doi.org/10.3233/RNN-2010-0533
Kim JS, Newman-Toker DE, Kerber KA, Jahn K, Bertholon P, Waterston J, Lee H, Bisdorff A, Strupp M (2022) Vascular vertigo and dizziness: diagnostic criteria. J Vestib Res 32(3):205–222. https://doi.org/10.3233/VES-210169
Tsivgoulis G, Palaiodimou L, Triantafyllou S, Köhrmann M, Dilaveris P, Tsioufis K, Magiorkinis G, Krogias C, Schellinger PD, Caso V, Paciaroni M, Sharma M, Lemmens R, Gladstone DJ, Sanna T, Wachter R, Filippatos G, Katsanos AH (2023) Prolonged cardiac monitoring for stroke prevention: a systematic review and meta-analysis of randomized-controlled clinical trials. Eur Stroke J 8(1):106–116. https://doi.org/10.1177/23969873221139410
Kim HA, Ahn J, Park HS, Lee SM, Choi SY, Oh EH, Choi JH, Kim JS, Choi KD (2021) Cardiogenic vertigo: characteristics and proposed diagnostic criteria. J Neurol 268(3):1070–1075. https://doi.org/10.1007/s00415-020-10252-4
Castellucci A, Malara P, Martellucci S, Delmonte S, Ghidini A (2021) Fluctuating posterior canal function in benign paroxysmal positional vertigo depending on how and where otoconia are disposed. Otol Neurotol 42(2):e193–e198. https://doi.org/10.1097/MAO.0000000000002913
Kabaya K, Katsumi S, Fukushima A, Esaki S, Minakata T, Iwasaki S (2023) Assessment of semicircular canal function in benign paroxysmal positional vertigo using the video head impulse test and caloric test. Laryngoscope Investig Otolaryngol 8(2):525–531. https://doi.org/10.1002/lio2.1020
Herdman SJ, Hall CD, Heusel-Gillig L (2020) Factors associated with rehabilitation outcomes in patients with unilateral vestibular hypofunction: a prospective cohort study. Phys Ther 100(11):2009–2022. https://doi.org/10.1093/ptj/pzaa138
Strupp M, Kim JS, Murofushi T, Straumann D, Jen JC, Rosengren SM, Della Santina CC, Kingma H (2017) Bilateral vestibulopathy: diagnostic criteria Consensus document of the Classification Committee of the Bárány Society. J Vestib Res 27(4):177–189. https://doi.org/10.3233/VES-170619 (Erratum.In:JVestibRes.2023;33(1):87)
Kim JS, Kim HJ (2022) Bilateral vestibulopathy: the causes, diagnosis, and treatments. Curr Opin Neurol 35(1):98–106. https://doi.org/10.1097/WCO.0000000000001014
Mancino-Moreira F, Rueda A, Esteban-Sanchez J, Martin-Sanz E (2021) Clinical subtypes and vHIT parameters in a population with bilateral vestibulopathy. Front Neurol 12:673974. https://doi.org/10.3389/fneur.2021.673974
Cohen HS, Sangi-Haghpeykar H (2020) Differences in responses on the modified clinical test of sensory interaction and balance on medium firm and medium density foam in healthy controls and patients with vestibular disorders. Biomed Hub 5(1):1548–1555. https://doi.org/10.1159/000507180
Herssens N, How D, van de Berg R, McCrum C (2022) Falls among people with bilateral vestibulopathy: a review of causes, incidence, injuries, and methods. JAMA Otolaryngol Head Neck Surg 148(2):187–192. https://doi.org/10.1001/jamaoto.2021.3673
Akdal G, Koçoğlu K, Bora E, Koç A, Ülgenalp A, Bedir M, Ala RT, Battaloğlu E, Kırkım G, Şengün İŞ, Halmágyi GM (2021) Selective bilateral vestibular neuropathy in a Turkish CMT1B family with a novel MPZ mutation. Neurol Clin Pract 11(2):e129–e134. https://doi.org/10.1212/CPJ.0000000000000930
Akdal G, Koçoğlu K, Tanrıverdizade T, Bora E, Bademkıran F, Yüceyar AN, Ekmekçi Ö, Şengün İŞ, Karasoy H (2021) Vestibular impairment in Charcot-Marie-Tooth disease. J Neurol 268(2):526–531. https://doi.org/10.1007/s00415-020-10186-x
Akdal G, Tanrıverdizade T, Şengün İ, Bademkıran F, Koçoğlu K, Yüceyar AN, Ekmekçi Ö, Karasoy H, Halmágyi GM (2018) Vestibular impairment in chronic inflammatory demyelinating polyneuropathy. J Neurol 265(2):381–387. https://doi.org/10.1007/s00415-017-8712-8
Ishai R, Seyyedi M, Chancellor AM, McLean CA, Rodriguez ML, Halmagyi GM, Nadol JB Jr, Szmulewicz DJ, Quesnel AM (2021) The pathology of the vestibular system in CANVAS. Otol Neurotol 42(3):e332–e340. https://doi.org/10.1097/MAO.0000000000002985
Davies K, Szmulewicz DJ, Corben LA, Delatycki M, Lockhart PJ (2022) RFC1-related disease: molecular and clinical insights. Neurol Genet 8(5):e200016. https://doi.org/10.1212/NXG.0000000000200016
van Stiphout L, Lucieer F, Pleshkov M, Van Rompaey V, Widdershoven J, Guinand N, Pérez Fornos A, Kingma H, van de Berg R (2022) Bilateral vestibulopathy decreases self-motion perception. J Neurol 269(10):5216–5228. https://doi.org/10.1007/s00415-021-10695-3
Bosmans J, Gommeren H, Mertens G, Cras P, Engelborghs S, Van Ombergen A, Vereeck L, Gilles A, Van Rompaey V (2022) Associations of bilateral vestibulopathy with cognition in older adults matched with healthy controls for hearing status. JAMA Otolaryngol Head Neck Surg 148(8):731–739. https://doi.org/10.1001/jamaoto.2022.1303
van Stiphout L, Pleshkov M, Lucieer F, Dobbels B, Mavrodiev V, Guinand N, Pérez Fornos A, Widdershoven J, Strupp M, Van Rompaey V, van de Berg R (2022) Patterns of vestibular impairment in bilateral vestibulopathy and its relation to etiology. Front Neurol 13:856472. https://doi.org/10.3389/fneur.2022.856472
van Dooren TS, Starkov D, Lucieer FMP, Vermorken B, Janssen AML, Guinand N, Pérez-Fornos A, Van Rompaey V, Kingma H, van de Berg R (2020) Comparison of three video head impulse test systems for the diagnosis of bilateral vestibulopathy. J Neurol 267(Suppl 1):256–264. https://doi.org/10.1007/s00415-020-10060-w
Pogson JM, Taylor RL, Bradshaw AP, McGarvie L, D’Souza M, Halmagyi GM, Welgampola MS (2019) The human vestibulo-ocular reflex and saccades: normal subjects and the effect of age. J Neurophysiol 122(1):336–349. https://doi.org/10.1152/jn.00847.2018
Agrawal Y, Van de Berg R, Wuyts F, Walther L, Magnusson M, Oh E, Sharpe M, Strupp M (2019) Presbyvestibulopathy: diagnostic criteria. Consensus document of the classification committee of the Bárány Society. J Vestib Res 29(4):161–170. https://doi.org/10.3233/VES-190672
Müller KJ, Becker-Bense S, Strobl R, Grill E, Dieterich M (2022) Chronic vestibular syndromes in the elderly: presbyvestibulopathy-an isolated clinical entity? Eur J Neurol 29(6):1825–1835. https://doi.org/10.1111/ene.15308
Eriksen ND, Hougaard DD (2023) Age- and gender-specific normative data on computerized dynamic posturography in a cohort of Danish adults. Eur Arch Otorhinolaryngol 280(5):2191–2200. https://doi.org/10.1007/s00405-022-07706-y
Teggi R, Familiari M, Battista RA, Gatti O, Cangiano I, Bussi M, Bubbico L (2023) The social problem of presbystasis and the role of vestibular rehabilitation in elderly patients: a review. Acta Otorhinolaryngol Ital. https://doi.org/10.14639/0392-100X-N1908. (Online ahead of print)
Arshad Q, Cousins S, Golding JF, Bronstein AM (2023) Factors influencing clinical outcome in vestibular neuritis—a focussed review and reanalysis of prospective data. J Neurol Sci 446:120579. https://doi.org/10.1016/j.jns.2023.120579
Pogson JM, Taylor RL, Bradshaw AP, McGarvie L, D’Souza M, Flanagan S, Kong J, Biggs N, Shivalingam B, Greenberg S, Croxson G, Halmagyi GM, Welgampola MS (2022) The human vestibulo-ocular reflex and compensatory saccades in schwannoma patients before and after vestibular nerve section. Clin Neurophysiol 138:197–213. https://doi.org/10.1016/j.clinph.2022.02.014
Taylor RL, Kong J, Flanagan S, Pogson J, Croxson G, Pohl D, Welgampola MS (2015) Prevalence of vestibular dysfunction in patients with vestibular schwannoma using video head-impulses and vestibular-evoked potentials. J Neurol 262(5):1228–1237. https://doi.org/10.1007/s00415-015-7697-4
Nilsen KS, Nordahl SHG, Berge JE, Dhayalan D, Goplen FK (2023) Vestibular tests related to tumor volume in 137 patients with small to medium-sized vestibular schwannoma. Otolaryngol Head Neck Surg. https://doi.org/10.1002/ohn.399. (In press)
Young AS, Taylor RL, McGarvie LA, Halmagyi GM, Welgampola MS (2016) Bilateral sequential peripheral vestibulopathy. Neurology 86(15):1454–1456. https://doi.org/10.1212/WNL.0000000000002563
Comacchio F, Mion M, Armato E, Castellucci A (2021) Sequential vestibular neuritis: report of four cases and literature review. J Audiol Otol 25(2):89–97. https://doi.org/10.7874/jao.2020.00360
Ahmed RM, Hannigan IP, MacDougall HG, Chan RC, Halmagyi GM (2012) Gentamicin ototoxicity: a 23-year selected case series of 103 patients. Med J Aust 196(11):701–704. https://doi.org/10.5694/mja11.10850
Akdal G, Koçoğlu K, Koçoğlu C, Bora E, Nazlı Başak A, Halmágyi GM (2021) Vestibulo-ocular reflex impairment in SPG7 hereditary spastic paraplegia. Clin Neurophysiol 132(1):77–79. https://doi.org/10.1016/j.clinph.2020.10.012
Wang A, Shearer AE, Zhou GW, Kenna M, Poe D, Licameli GR, Brodsky JR (2021) Peripheral vestibular dysfunction is a common occurrence in children with non-syndromic and syndromic genetic hearing loss. Front Neurol 12:714543. https://doi.org/10.3389/fneur.2021.714543
Delmaghani S, El-Amraoui A (2022) The genetic and phenotypic landscapes of Usher syndrome: from disease mechanisms to a new classification. Hum Genet 141(3–4):709–735. https://doi.org/10.1007/s00439-022-02448-7
Halmagyi GM, Parker GD, Chen L, Welgampola MS, Watson JDG, Barnett MH, Todd MJ, El-Wahsh S, Rose V, Stoodley MA, Brennan JW (2023) Progressive loss of hearing and balance in superficial siderosis due to occult spinal dural defects. Eur Arch Otorhinolaryngol 280(2):633–641. https://doi.org/10.1007/s00405-022-07523-3
Pollak L, Milo R, Kossych V, Rabey MJ, Shapira E (2001) Bilateral vestibular failure as a unique presenting sign in carcinomatous meningitis: case report. J Neurol Neurosurg Psychiatry 70(5):704–705. https://doi.org/10.1136/jnnp.70.5.704
Halmágyi GM, Szmulewicz DJ (2021) Vestibular function testing in patients with RFC1 mutations. J Neurol 268(12):4894–4896. https://doi.org/10.1007/s00415-021-10698-0 (Erratum in: J Neurol 2022;269(4):2264. https://doi.org/10.1007/s00415-022-10975-6)
Taylor RL, Antunovich T, Chang TMH, Rodrigues M, Baker A, Bergin P, McGuinness B, Roxburgh RH (2023) Hyperactive vestibular and visually enhanced vestibulo-ocular reflexes in autosomal recessive cerebellar ataxia type 3: a case report. J Neurol 270(2):1154–1158. https://doi.org/10.1007/s00415-022-11422-2
Mantokoudis G, Saber Tehrani AS, Kattah JC, Eibenberger K, Guede CI, Zee DS, Newman-Toker DE (2015) Quantifying the vestibulo-ocular reflex with video-oculography: nature and frequency of artifacts. Audiol Neurootol 20(1):39–50. https://doi.org/10.1159/000362780
Migliaccio AA, Cremer PD (2011) The 2D modified head impulse test: a 2D technique for measuring function in all six semi-circular canals. J Vestib Res 21(4):227–234. https://doi.org/10.3233/VES-2011-0421
MacDougall HG, McGarvie LA, Halmagyi GM, Rogers SJ, Manzari L, Burgess AM, Curthoys IS, Weber KP (2016) A new saccadic indicator of peripheral vestibular function based on the video head impulse test. Neurology 87(4):410–418. https://doi.org/10.1212/WNL.0000000000002827
Baloh RW (2002) Robert Bárány and the controversy surrounding his discovery of the caloric reaction. Neurology 58(7):1094–1099. https://doi.org/10.1212/wnl.58.7.1094
Starkov D, Strupp M, Pleshkov M, Kingma H, van de Berg R (2021) Diagnosing vestibular hypofunction: an update. J Neurol 268(1):377–385. https://doi.org/10.1007/s00415-020-10139-4
van Esch BF, Nobel-Hoff GE, van Benthem PP, van der Zaag-Loonen HJ, Bruintjes TD (2016) Determining vestibular hypofunction: start with the video-head impulse test. Eur Arch Otorhinolaryngol 273(11):3733–3739. https://doi.org/10.1007/s00405-016-4055-9
Molnár A, Maihoub S, Tamás L, Szirmai Á (2023) Comparison between caloric and video-head impulse tests in Ménière’s disease and vestibular neuritis. Int J Audiol 62(5):393–399. https://doi.org/10.1080/14992027.2022.2059711
Tsuji K, Velázquez-Villaseñor L, Rauch SD, Glynn RJ, Wall C 3rd, Merchant SN (2000) Temporal bone studies of the human peripheral vestibular system. Meniere’s disease. Ann Otol Rhinol Laryngol Suppl 181:26–31. https://doi.org/10.1177/00034894001090s505
Shen LL, Andresen NS, Chari DA, Pogson JM, Lauer AM, Rabbitt RD, Carey JP, Santos F, Ward BK (2023) Otolith membrane herniation, not semicircular canal duct dilation, is associated with decreased caloric responses in Ménière’s disease. J Assoc Res Otolaryngol 24(1):95–106. https://doi.org/10.1007/s10162-022-00883-x
Bartolomeo M, Biboulet R, Pierre G, Mondain M, Uziel A, Venail F (2014) Value of the video head impulse test in assessing vestibular deficits following vestibular neuritis. Eur Arch Otorhinolaryngol 271(4):681–688. https://doi.org/10.1007/s00405-013-2451-y
Curthoys IS (2012) The interpretation of clinical tests of peripheral vestibular function. Laryngoscope 122(6):1342–1352. https://doi.org/10.1002/lary.23258
Colebatch JG, Rosengren SM (2016) Safe levels of acoustic stimulation for VEMPs: comment on “Sudden bilateral hearing loss after cervical and ocular vestibular evoked myogenic potentials.” Otol Neurotol 37(1):117–118. https://doi.org/10.1097/MAO.0000000000000912
Zuniga MG, Schell A, Engst BG, Carey JP (2021) Reducing sound exposure during ocular vestibular evoked myogenic potential testing for superior semicircular canal dehiscence syndrome. Otol Neurotol 42(6):e735–e743. https://doi.org/10.1097/MAO.0000000000003084
Papathanasiou ES, Straumann D (2019) Why and when to refer patients for vestibular evoked myogenic potentials: a critical review. Clin Neurophysiol 130(9):1539–1556. https://doi.org/10.1016/j.clinph.2019.04.719
Castellucci A, Piras G, Del Vecchio V, Ferri GG, Ghidini A, Brandolini C (2021) Which inner ear disorders lie behind a selective posterior semicircular canal hypofunction on video head impulse test? Otol Neurotol 42(4):573–584. https://doi.org/10.1097/MAO.0000000000002995
Janky KL, Nguyen KD, Welgampola M, Zuniga MG, Carey JP (2013) Air-conducted oVEMPs provide the best separation between intact and superior canal dehiscent labyrinths. Otol Neurotol 34(1):127–134. https://doi.org/10.1097/MAO.0b013e318271c32a
Curthoys IS, Burgess AM, Manzari L, Pastras CJ (2022) A single fast test for semicircular canal dehiscence—oVEMP n10 to 4000 Hz—depends on stimulus rise time. Audiol Res 12(5):457–465. https://doi.org/10.3390/audiolres12050046
Kim DH, Kim SW, Kim SH, Jung JH, Hwang SH (2022) Usefulness of cervical vestibular-evoked myogenic potentials for diagnosing patients with superior canal dehiscence syndrome: a meta-analysis. Otol Neurotol 43(2):146–152. https://doi.org/10.1097/MAO.0000000000003430
Ward BK, van de Berg R, van Rompaey V, Bisdorff A, Hullar TE, Welgampola MS, Carey JP (2021) Superior semicircular canal dehiscence syndrome: diagnostic criteria consensus document of the committee for the classification of vestibular disorders of the Bárány Society. J Vestib Res 31(3):131–141. https://doi.org/10.3233/VES-200004
Purcell I, Qu Y (2018) DizzyDoctor system. https://www.dizzydoctor.com/. Accessed 7 June 2023
Phillips JS, Newman JL, Cox SJ (2019) An investigation into the diagnostic accuracy, reliability, acceptability and safety of a novel device for Continuous Ambulatory Vestibular Assessment (CAVA). Sci Rep 9(1):10452. https://doi.org/10.1038/s41598-019-46970-7
Phillips JS, Newman J, Cox S (2021) Towards providing an automated approach to differentiating the nystagmus of Ménière’s disease, vestibular migraine, and benign paroxysmal positional vertigo. Otol Neurotol 42(6):890–896. https://doi.org/10.1097/MAO.0000000000003083
Beam AL, Drazen JM, Kohane IS, Leong TY, Manrai AK, Rubin EJ (2023) Artificial Intelligence in Medicine. N Engl J Med 388(13):1220–1221. https://doi.org/10.1056/NEJMe2206291
Kabade V, Hooda R, Raj C, Awan Z, Young AS, Welgampola MS, Prasad M (2021) Machine learning techniques for differential diagnosis of vertigo and dizziness: a review. Sensors (Basel) 21(22):7565. https://doi.org/10.3390/s21227565
Yu F, Wu P, Deng H, Wu J, Sun S, Yu H, Yang J, Luo X, He J, Ma X, Wen J, Qiu D, Nie G, Liu R, Hu G, Chen T, Zhang C, Li H (2022) A Questionnaire-based ensemble learning model to predict the diagnosis of vertigo: model development and validation study. J Med Internet Res 24(8):e34126. https://doi.org/10.2196/34126
Lim EC, Park JH, Jeon HJ, Kim HJ, Lee HJ, Song CG, Hong SK (2019) Developing a diagnostic decision support system for benign paroxysmal positional vertigo using a deep-learning model. J Clin Med 8(5):633. https://doi.org/10.3390/jcm8050633
Lu W, Li Z, Li Y, Li J, Chen Z, Feng Y, Wang H, Luo Q, Wang Y, Pan J, Gu L, Yu D, Zhang Y, Shi H, Yin S (2022) A deep learning model for three-dimensional nystagmus detection and its preliminary application. Front Neurosci 16:930028. https://doi.org/10.3389/fnins.2022.930028
Wagle N, Morkos J, Liu J, Reith H, Greenstein J, Gong K, Gangan I, Pakhomov D, Hira S, Komogortsev OV, Newman-Toker DE, Winslow R, Zee DS, Otero-Millan J, Green KE (2022) aEYE: a deep learning system for video nystagmus detection. Front Neurol 13:963968. https://doi.org/10.3389/fneur.2022.963968
Wu P, Liu X, Dai Q, Yu J, Zhao J, Yu F, Liu Y, Gao Y, Li H, Li W (2023) Diagnosing the benign paroxysmal positional vertigo via 1D and deep-learning composite model. J Neurol. https://doi.org/10.1007/s00415-023-11662-w. (In press)
Zhang W, Wu HY, Liu Y, Zheng S, Liu ZZ, Li YR, Zhao Y, Zhu ZF (2021) Deep learning based torsional nystagmus detection for dizziness and vertigo diagnosis. Biomed Signal Proc Control 68:102616. https://doi.org/10.1016/j.bspc.2021.102616
Korda A, Wimmer W, Wyss T, Michailidou E, Zamaro E, Wagner F, Caversaccio MD, Mantokoudis G (2022) Artificial intelligence for early stroke diagnosis in acute vestibular syndrome. Front Neurol 13:919777. https://doi.org/10.3389/fneur.2022.919777
Bragg PG, Norton BM, Petrak MR, Weiss AD, Kandl LM, Corrigan ML, Bahner CL, Matsuoka AJ (2023) Application of supervised machine learning algorithms for the evaluation of utricular function on patients with Meniere’s disease: utilizing subjective visual vertical and ocular-vestibular-evoked myogenic potentials. Acta Otolaryngol 143(4):262–273. https://doi.org/10.1080/00016489.2023.2190163
Miettinen K, Juhola M (2010) Classification of otoneurological cases according to Bayesian probabilistic models. J Med Syst 34(2):119–130. https://doi.org/10.1007/s10916-008-9223-z
Vivar G, Strobl R, Grill E, Navab N, Zwergal A, Ahmadi SA (2021) Using base-ml to learn classification of common vestibular disorders on DizzyReg Registry data. Front Neurol 12:681140. https://doi.org/10.3389/fneur.2021.681140
Kim BJ, Jang SK, Kim YH, Lee EJ, Chang JY, Kwon SU, Kim JS, Kang DW (2021) Diagnosis of acute central dizziness with simple clinical information using machine learning. Front Neurol 12:691057. https://doi.org/10.3389/fneur.2021.691057
Ahmadi SA, Vivar G, Navab N, Möhwald K, Maier A, Hadzhikolev H, Brandt T, Grill E, Dieterich M, Jahn K, Zwergal A (2020) Modern machine-learning can support diagnostic differentiation of central and peripheral acute vestibular disorders. J Neurol 267(Suppl 1):143–152. https://doi.org/10.1007/s00415-020-09931-z
Groezinger M, Huppert D, Strobl R, Grill E (2020) Development and validation of a classification algorithm to diagnose and differentiate spontaneous episodic vertigo syndromes: results from the DizzyReg patient registry. J Neurol 267(Suppl 1):160–167. https://doi.org/10.1007/s00415-020-10061-9
Newman JL, Phillips JS, Cox SJ (2021) 1D convolutional neural networks for detecting nystagmus. IEEE J Biomed Health Inform 25(5):1814–1823. https://doi.org/10.1109/JBHI.2020.3025381
Challen R, Denny J, Pitt M, Gompels L, Edwards T, Tsaneva-Atanasova K (2019) Artificial intelligence, bias and clinical safety. BMJ Qual Saf 28(3):231–237. https://doi.org/10.1136/bmjqs-2018-008370
Parasuraman R, Manzey DH (2010) Complacency and bias in human use of automation: an attentional integration. Hum Factors 52(3):381–410. https://doi.org/10.1177/0018720810376055
Acknowledgement
Without a supreme editorial effort from Dr. Ann Burgess this manuscript would not have seen the light of day.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Halmágyi, G.M., Akdal, G., Welgampola, M.S. et al. Neurological update: neuro-otology 2023. J Neurol 270, 6170–6192 (2023). https://doi.org/10.1007/s00415-023-11922-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-11922-9