Abstract
Background
This study aimed to summarize the clinical features of Autoimmune Glial Fibrillary Acidic Protein Astrocytosis mimicking tuberculosis meningitis to improve clinicians’ understanding of this disease.
Methods
We retrospectively analyzed the clinical manifestations, cerebrospinal fluid results, and imaging data of five patients with Autoimmune Glial Fibrillary Acidic Protein Astrocytosis mimicking tuberculous meningitis who were admitted to Xiangya Hospital Central South University between October 2021 and July 2022.
Results
Five patients were aged 31–59 years, with a male-to-female ratio of 4:1. Among the cases reviewed, four had a history of prodromal infections manifesting as fever and headache. One patient developed limb weakness and numbness with clinical manifestations of meningitis, meningoencephalitis, encephalomyelitis, or meningomyelitis. Cerebrospinal fluid analysis revealed an increased cell count in five cases, with a lymphocyte majority. All five cases had a CSF protein level > 1.0 g/L, CSF/blood glucose ratio < 0.5, and two patients had CSF glucose < 2.2 mmol/L. Decreased CSF chloride was observed in three cases, while increased ADA was observed in one case. Both serum and cerebrospinal fluid were positive for anti-GFAP antibodies in three cases, while in two cases, only CSF was positive for anti-GFAP antibodies. Additionally, hyponatremia and hypochloremia were observed in three cases. No tumors were detected in any of the five patients during tumor screening, and all five cases had a good prognosis following immunotherapy.
Conclusion
Anti-GFAP antibody testing should be routinely performed in patients with suspected tuberculosis meningitis to avoid misdiagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autoimmune glial fibrillary acidic protein astrocytopathy (A-GFAP-A) is a group of autoimmune neurological diseases [1, 2] affecting the brain, meninges, spinal cord, and optic nerve. GFAP is an intermediate filament protein essential for the cytoskeletal structure of adult astrocytes. In 2016, FANG et al. [1] reported that GFAP-IgG in the cerebrospinal fluid (CSF) and/or serum by cell-based testing could be a potential biomarker for diagnosing this disease. Clinical symptoms include fever, headache, brain and meningeal abnormalities, imaging and cerebrospinal fluid changes, lack of specificity, and susceptibility to misdiagnosis. Especially when the cerebrospinal fluid depicts tuberculous lesions, it is easily confused with tuberculous meningitis (TBM) [3,4,5,6,7,8,9]. This study retrospectively examined the clinical features of A-GFAP-A in patients with tuberculous meningitis to improve understanding and diagnostic efficacy.
Materials and methods
Research objects
This research retrospectively analyzed five patients with A-GFAP-A and clinical features of tuberculous meningitis who were admitted to the Neurology Department of Xiangya Hospital of Central South University between October 2021 and July 2022. The patients satisfied the following inclusion criteria[1]: (1) The clinical manifestations were similar to those of encephalitis, meningitis, myelitis, encephalomyelitis, or meningomyelitis; (2) all patients underwent serum and/or CSF testing, and all of them tested positive for CSF GFPA antibodies through cell-based assay (CBA).
Research methodology
Data collection
The collected and analyzed clinical data included age, gender, first symptoms, main clinical manifestations, laboratory tests, imaging tests, treatment plans, and follow-up prognoses by phone.
Cell‑based assay
This study used a cell-based assay (CBA) method because of its high specificity and sensitivity. CBA is a cell transfection-based indirect immunofluorescence assay. In this assay, GFAP genes are transfected into mammalian cells to express GFAP antigens in the mammalian cytoplasm. Green fluorescent proteins (GFP) were also expressed in the transfection as an internal reference for detection. The transfected cells were then fixed onto 96-well microplates to make antigen plates. GFAP antibodies in human serum, plasma, and cerebrospinal fluid samples were detected semi-quantitatively using indirect immunofluorescence.
The autoantibodies were detected using the CBA method. Briefly, the serum to be tested was diluted 1:10, or the original fluid of cerebrospinal fluid was used to incubate the patch of cells at room temperature for 60 min. The patch of cells was then treated with detergent three times, for five min each. The FITC-labeled goat anti-human IgG secondary antibody was then diluted 1:50 and incubated in the cell patch for 30 min at room temperature. The patch of cells was then cleansed, sealed, and observed under a fluorescence microscope. The fluorescence signal was significantly higher than the background signal, allowing the serum in the cell membrane to be identified as the positive serum of autoantibodies. When testing the titer of the positive serum, the serum or cerebrospinal fluid was diluted in a gradient, and the method described above was followed. The titer value of the positive serum was determined as the highest dilution multiple of the detectable positive signal.
Prognostic assessment
The prognosis was assessed using the modified Rankin Scale (mRS). An mRS score of 0 ~ 2 was considered a good prognosis, whereas a score of > 2 was considered poor.
Results
Clinical features
The clinical characteristics are summarized in Table 1. The reviewed cases included four males and one female. All patients reported subacute disease with clinical manifestations consistent with meningoencephalitis, meningitis, encephalomyelitis, or meningomyelitis. In four cases, acute meningeal symptoms manifested as neck stiffness. Prodromal infections, fever, and headache were observed in four patients, respectively. There were signs of cognitive function decline in three of the reviewed cases. Urinary and fecal passage difficulties were observed in two patients. One patient exhibited vomiting, slurred speech, limb tremors, unsteady walking, lower extremities weakness, limb weakness and numbness, autonomic system dysfunction, and intestinal paralysis.
Lab tests
-
(1)
Cerebrospinal fluid (CSF) findings: Three patients had normal cerebrospinal fluid pressure, while the other two were presented with elevated pressure (reference range: 80–180 mmHg). High white blood cells count (reference range: 0–10 × 106/L), predominantly lymphocytes, was observed in five patients. Elevated CSF protein levels with values > 1.0 g/L (reference range: 0.15–0.45 g/L) were observed in all five cases. The CSF/blood glucose ratio < 0.5 was observed in all five cases, and in three cases, the absolute CSF glucose level (reference range: 2.5–4.4 mmol/L) was reduced. All five patients had reduced CSF chloride levels (reference range: 120–130 mmol/L), while one patient had elevated adenosine dehydrogenase (ADA) (reference range: 0–4 U/L). CSF immunoglobulin studies revealed that coxsackie virus IgM and human herpesvirus IgM were positive in one case, respectively. All five patients were negative for CSF mycobacterium tuberculosis smears, CSF mycobacterium tuberculosis cultures, CSF mycobacterium tuberculosis antibodies, CSF bacteriological culture, and CSF fungal culture (Table 2).
-
(2)
Blood serum tests: Among the patients included, three had hyponatremia (reference range: 137–147 mmol/L) and hypochlorhydria (reference range: 99–110 mmol/L, Table 2).
-
(3)
Antibody test: Positive GFAP serum and CSF antibodies were observed in three cases, while in the remaining two cases, it was only positive in CSF. Weakly positive anti-amphiphysin, anti-SSA, and anti-Ro-52 antibodies were detected in the pooled serum of one patient. Both serum and CSF were negative for MOG and AQP4 antibodies in all five cases.
-
(4)
Other tests: The tuberculosis diagnostic tests, i.e., T-spot and PPD skin test, were negative for all patients. Pulmonary CT, HIV, serum Treponema pallidum hemagglutination test (TPHA), serum tumor marker, and thyroid function tests did not reveal any abnormalities.
MRI findings
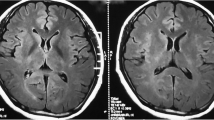
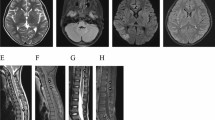
The results of MRI findings are displayed in Table 3. All five patients received Brain MRI scans for enhancement but did not exhibit any enhancement. Among them, the scan of case 1 revealed bilateral basal ganglia and left corpus callosum abnormalities on both T1- and T2-weighted images, with high signals being revealed on the Flair image (Fig. 1). Case 5 revealed multiple T1 and T2 patchy signal abnormalities in the bilateral basal ganglia, thalamus, and right corpus callosum (Fig. 2), whereas the other three cases demonstrated no anomalies. Spinal MRI scan enhancement was performed on three patients. Among them, case 4 displayed strip and sheet-shaped equal T1 and long T2 signals in the horizontal spinal cord from the 2nd cervical vertebra to the 1st lumbar vertebrae without enhancement, primarily affecting the central part of the spinal cord (Fig. 3). Case 5 revealed a slightly thickened spinal cord in the cervicothoracic segment, in which the slightly long T1 and T2 signals could be observed (Fig. 2). These signals were mainly located in the central part of the spinal cord. After enhancement, the spot and sheet-shaped signals were enhanced. No abnormal occurrences were observed in case 1.
Brain MRI showing T2 patchy signal abnormalities in the bilateral basal ganglia, thalamus, and right corpus callosum, with the Flair image revealing high signals. (A, B). A slightly thickened spinal cord in the cervicothoracic segment, in which the slightly long T2 signals could be seen (C, D). These signals were mainly in the central part of the spinal cord (E)
Electrophysiological test
Video EEG tests were performed in three cases, one of which revealed diffuse slow waves.
Treatment and outcome
In the acute stage, all patients reviewed in this study received either glucocorticoid shock therapy or combination therapy with plasma exchange, and all of them responded well to treatment, as evidenced by the reduced mRS scores. The follow-up period was from the 2nd to the 8th month, with an average follow-up time of four months. During the most recent follow-up, the mRS scores of four cases were less than 2, while the mRS score of the remaining cases was 3, but without recurrence. There was no evidence of tumor recurrence in any reviewed patients during the follow-up period.
Discussion
In this study, all five patients reviewed had a subacute disease, with fever and headache being the first symptoms for four patients. The primary clinical syndromes were meningitis, meningoencephalitis, encephalomyelitis, or meningomyelitis. Pulmonary CT, T-SPOT, PPD skin test, CSF mycobacterium tuberculosis cultures, CSF bacteriological culture, CSF fungal culture, HIV, TPHA, and serum tumor marker were negative for all patients. Their cerebrospinal fluid analysis revealed increased cell counts, with predomination of lymphocytes, elevated CSF proteins > 1.0 g/L, and CSF/blood glucose ratio < 0.5. All cases tested positive for GFAP antibody in CSF, and their response to corticosteroid therapy was excellent, confirming the diagnosis of A-GFAP-A. The clinical symptoms, cranial imaging changes, and other CSF anomalies of the five A-GFAP-A patients reported in this study were similar to those of tuberculous meningitis (TBM). According to previous studies, the misdiagnosis rate ranges between 4.5% and 35.7% [3, 4]. The presence of mycobacterium tuberculosis in cerebrospinal fluid is required for a definitive diagnosis of TBM. However, the pathological identification of mycobacterium sp. in CSF is clinically challenging; consequently, it is primarily diagnosed based on clinical symptoms, cranial imaging changes, other CSF anomalies, and response to diagnostic antituberculosis therapy, particularly in TB-endemic regions.
In this study, all five patients underwent brain MRI scans enhancement and demonstrated no enhancement. The brain MRI images of two patients depicted abnormal T2-weighted and FLAIR imaging of hyperintensity signals in the bilateral basal ganglia. Moreover, the brain MRI images of one patient exhibited abnormal T2-weighted and FLAIR imaging of hyperintensity signals in the bilateral thalamus lesion. Although previous studies indicate that linear perivascular radial gadolinium enhancement in the brain is a characteristic manifestation of the disease [2, 4, 10, 11], it has been suggested that radial perivascular emphasis is not necessarily associated with GFAP antibodies [12]. In this study, none of the five patients demonstrated linear perivascular radial gadolinium enhancement of the brain. Previous studies have reported that abnormal high-head MR signals in A-GFAP-A and TBM patients can involve the basal ganglia and thalamus [2,3,4,5, 10, 13,14,15]. Kimura et al. [4] even suggested that the bilateral thalamus abnormal hyperintense signal was the defining feature of A-GFAP-A. Zhuang et al. [10] depicted that patients with A-GFAP-A who had a high signal in the bilateral thalamus, basal ganglia region, and periventricular white matter accumulated inflammatory cells, antigens, and antibodies in the perivascular and V-R spaces. In cases with typical brain MRI findings (perivascular radial gadolinium enhancement), clinicians may easily suspect A-GFAP-A. However, in patients without a typical brain MRI finding, T2WI/FLAIR lesions involving bilateral basal ganglia and thalamus may make it difficult to differentiate between these two diseases. The rapid disappearance of brain MRI abnormalities in A-GFAP-A after corticosteroid administration may be a distinctive feature [16]. TBM can only resolve after anti-TB treatment. Therefore, the treatment efficacy may be a differentiating factor.
In this study, the similarities and differences between A-GFAP-A and TBM were primarily discussed regarding cerebrospinal fluid changes to improve the understanding of clinicians about the disease. In this study, five cases had elevated CSF cell counts, with lymphocytes predominating. Elevated CSF proteins > 1.0 g/L were also observed in all five cases. Elevated CSF lymphocytes, protein is more common in TBM; autoimmune disease is uncommon. Studies have revealed that the cerebrospinal fluid of A-GFAP-A patients is predominantly characterized by inflammatory changes and that CSF lymphocytes and proteins can be increased, with some patients exhibiting CSF protein > 1.0 g/L [2, 4,5,6, 8,9,10]. A CSF protein threshold of > 1.0 g/L (100 mg/dL) differentiated between cases of TBM, bacterial meningitis[17], and viral meningitis [18]. Solomons et al. [19] demonstrated that CSF protein > 1.0 g/L has a sensitivity of 78% and specificity of 94% for diagnosing TBM. In this study, CSF protein levels in all five cases were > 1.0 g/L, indicating that elevated CSF protein levels are not unique to TBM diagnosis. According to this study, a mild increase in CSF WBC in patients with A-GFAP-A was usually mismatched with a significant increase in CSF protein. CSF WBCs and CSF protein were concurrently significantly increased in patients with TBM. This may be one of the distinguishing factors between the two.
In this study, the CSF/ blood glucose ratio in all five cases was < 0.5, and two patients had CSF glucose < 2.2 mmol/L. The patient demonstrated decreased CSF glucose but normal serum glucose, which is hardly seen in autoimmune diseases. Studies have shown that the decrease in CSF glucose in A-GFP-A patients is only 15–18% [2, 13]. Reduced CSF glucose has been reported to be more prevalent in TBM. Solomons et al. [19] reported that a CSF glucose concentration of < 2.2 mmol/L diagnosed TBM with a sensitivity of 68% and a specificity of 96%. The sensitivity using a CSF to serum glucose ratio of < 0.5 was 90%. In a study by Jipa et al. [20], 90.3% of their TBM patients had CSF/blood glucose ratios < 0.5. Since CSF glucose concentration is highly dependent on blood glucose concentration, the CSF/ blood glucose ratio is more significant in the differential diagnosis to exclude blood factors. This study observed a similar change, which prompted the specificity and sensitivity not to be unique to TBM, necessitating the use of A-GFAP-A as a possible differential diagnosis. The decrease of CSF glucose in TBM patients may be due to the release of glycolytic enzymes from the brain and the consumption of glucose, whereas the cause of the decrease of glucose in A-GFAP-A patients remains unknown.
In this study, one patient experienced a transient increase in CSF adenosine deaminase (ADA) activity, which recovered spontaneously. Kimura et al. [4] reported that 71.4% of A-GFAP-A patients had a transient increase in ADA level within the first month of onset, which they regarded as a unique feature of the CSF of early A-GFAP-A patients. Nakamura et al. [21] reported elevated ADA levels in the cerebrospinal fluid without mycobacterium tuberculosis infection in support of A-GFAP-A diagnosis. ADA is essential in the growth and differentiation of lymphocytes and macrophages. Kimura et al. [4] reported that elevated ADA levels might be associated with immunological pathogenesis during the early stage of A-GFAP-A. Changes in ADA levels in the CSF of one patient in this study were similar to those described in the literature, with a transient increase in ADA followed by spontaneous recovery. This spontaneous recovery contrasts with the elevated CSF ADA found in TBM patients who only recover after antituberculosis therapy.
We reported five cases of A-GFAP-A with a clinical presentation and CSF profile resembling TB meningitis. We consider A-GFAP-A an important differential diagnosis, particularly in TB-endemic regions. In clinical practice, CSF GFAP-igg should be considered for early evaluation in patients with suspected tuberculous meningoencephalitis and atypical characteristics, and diagnostic antituberculosis should be administered with caution.
Availability of data and materials
The corresponding author has full access to all data and has the right to publish any and all data separate and apart from any sponsor.
References
Fang B et al (2016) Autoimmune glial fibrillary acidic protein astrocytopathy: a novel meningoencephalomyelitis. JAMA Neurol 73(11):1297–1307
Flanagan EP et al (2017) Glial fibrillary acidic protein immunoglobulin G as biomarker of autoimmune astrocytopathy: Analysis of 102 patients. Ann Neurol 81(2):298–309
Iorio R et al (2018) Clinical and immunological characteristics of the spectrum of GFAP autoimmunity: a case series of 22 patients. J Neurol Neurosurg Psychiatry 89(2):138–146
Kimura A et al (2019) Clinical characteristics of autoimmune GFAP astrocytopathy. J Neuroimmunol 332:91–98
Ip B et al (2020) Autoimmune glial fibillary acidic protein astrocytopathy associated meningoencephalomyelitis and bilateral sensorineuronal deafness. Mult Scler Relat Disord 40:101922
Yang X et al (2020) Autoimmune glial fibrillary acidic protein astrocytopathy mimics infectious meningitis: Two case reports. Mult Scler Relat Disord 45:102350
Issa N et al (2020) Severe anti-GFAP meningo-encephalomyelitis following viral infection. Mult Scler Relat Disord 45:102448
Quek AM et al (2022) Autoimmune glial fibrillary acidic protein astrocytopathy masquerading as tuberculosis of the central nervous system: a case series. Int J Infect Dis 124:164–167
Someko H, Shiojiri T (2022) Autoimmune glial fibrillar acidic protein astrocytopathy mimicking tuberculous meningitis. BMJ Case Rep 15(11):e252518
Zhuang X et al (2022) Autoimmune glial fibrillary acidic protein astrocytopathy in children: a retrospective study. Eur J Med Res 27(1):11
Long Y et al (2018) Autoimmune glial fibrillary acidic protein astrocytopathy in Chinese patients: a retrospective study. Eur J Neurol 25(3):477–483
Wickel J et al (2020) Encephalitis with radial perivascular emphasis: Not necessarily associated with GFAP antibodies. Neurol Neuroimmunol Neuroinflamm 7(2):e670
Gravier-Dumonceau A et al (2022) Glial fibrillary acidic protein autoimmunity: a french cohort study. Neurology 98(6):e653–e668
Wasay M et al (2018) Frequency and impact of cerebral infarctions in patients with tuberculous meningitis. Stroke 49(10):2288–2293
Marie CC, Sy AI, Espiritu JL, Pascual R (2022) Global frequency and clinical features of stroke in patients with tuberculous meningitis: a systematic review. JAMA Netw Open 5(9):e2229282
Liao H et al (2022) MRI features and evolution of autoimmune glial fibrillary acidic protein astrocytopathy: a retrospective cross-sectional and longitudinal study. Mult Scler Related Disord 58:103512
Youssef FG et al (2006) Differentiation of tuberculous meningitis from acute bacterial meningitis using simple clinical and laboratory parameters. Diagn Microbiol Infect Dis 55(4):275–278
Hristea A et al (2012) Clinical prediction rule for differentiating tuberculous from viral meningitis. Int J Tuberc Lung Dis 16(6):793–798
Solomons RS et al (2015) The diagnostic value of cerebrospinal fluid chemistry results in childhood tuberculous meningitis. Childs Nerv Syst 31(8):1335–1340
Jipa R et al (2017) Rapid clinical score for the diagnosis of tuberculous meningitis: a retrospective cohort study. Ann Indian Acad Neurol 20(4):363–366
Nakamura S et al (2021) Self-remitting elevation of adenosine deaminase levels in the cerebrospinal fluid with autoimmune glial fibrillary acidic protein astrocytopathy: a case report and review of the literature. Intern Med 60(18):3031–3036
Funding
This work was supported by grants from the National Natural Science Foundation of China (No.82160420), Guangxi Province Health Committee self-funded project (No. Z20210494), the Clinical Climbing Program of the First Affiliated Hospital of Guangxi Medical University (No. YYZS2020022), Open Project of Guangxi Key Laboratory of Regenerative Medicine, Guangxi Medical University (No.202203), and “Medical Excellence Award” Funded by the Creative Research Development Grant from the First Affiliated Hospital of Guangxi Medical University.
Author information
Authors and Affiliations
Contributions
YL conducted the literature review and drafted the manuscript. GQ and BL reviewed and revised the manuscript. GL and HZ were responsible for revising the manuscript critically and have given final approval of the version to be published. All the authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical approval
The studies involving human participants were reviewed and approved by the Ethics Committee of Xiangya Hospital of Central South University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the patient for the publication of any potentially identifiable images or data included in this article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liang, Y., Wang, G., Li, B. et al. Autoimmune glial fibrillary acidic protein astrocytosis mimicking tuberculous meningitis: a retrospective study. J Neurol 270, 4860–4867 (2023). https://doi.org/10.1007/s00415-023-11818-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-11818-8