Abstract
Background
The in vivo relation between microglia activation and demyelination in multiple sclerosis is still unclear.
Objective
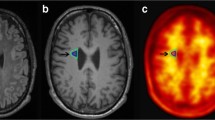
We combined 11C-PBR28 positron emission tomography and rapid estimation of myelin for diagnostic imaging (REMyDI) to characterize the relation between these pathological processes in a heterogeneous MS cohort.
Methods
11C-PBR28 standardized uptake values normalized by a pseudo-reference region (SUVR) were used to measure activated microglia. A voxelwise analysis compared 11C-PBR28 SUVR in the white matter of 38 MS patients and 16 matched healthy controls. The relative difference in SUVR served as a threshold to classify patients’ lesioned, perilesional and normal-appearing white matter as active or inactive. REMyDI was acquired in 27 MS patients for assessing myelin content in active and inactive white matter and its relationship with SUVR. Finally, we investigated the contribution of radiological metrics to clinical outcomes.
Results
11C-PBR28 SUVR were abnormally higher in several white matter areas in MS. Myelin content was lower in active compared to inactive corresponding white matter regions. An inverse correlation between SUVR and myelin content was found. Radiological metrics correlated with both neurological and cognitive impairment.
Conclusion
our data suggest an inverse relation of microglia activation and myelination, particularly in perilesional white matter tissue.




Similar content being viewed by others
References
Dal Bianco A, Grabner G, Kronnerwetter C, Weber M, Höftberger R, Berger T, Auff E, Leutmezer F, Trattnig S, Lassmann H, Bagnato F, Hametner S (2017) Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathol 133(1):25–42. https://doi.org/10.1007/s00401-016-1636-z
Dal Bianco A, Grabner G, Kronnerwetter C, Weber M, Kornek B, Kasprian G, Berger T, Leutmezer F, Rommer PS, Trattnig S, Lassmann H, Hametner S (2021) Long-term evolution of multiple sclerosis iron rim lesions in 7 T MRI. Brain 144(3):833–847. https://doi.org/10.1093/brain/awaa436
Banati RB, Newcombe J, Gunn RN, Cagnin A, Turkheimer F, Heppner F, Price G, Wegner F, Giovannoni G, Miller DH, Perkin GD, Smith T, Hewson AK, Bydder G, Kreutzberg GW, Jones T, Cuzner ML, Myers R (2000) The peripheral benzodiazepine binding site in the brain in multiple sclerosis: quantitative in vivo imaging of microglia as a measure of disease activity. Brain 123(Pt 11):2321–2337. https://doi.org/10.1093/brain/123.11.2321
Oh U, Fujita M, Ikonomidou VN, Evangelou IE, Matsuura E, Harberts E, Fujimura Y, Richert ND, Ohayon J, Pike VW, Zhang Y, Zoghbi SS, Innis RB, Jacobsonet S (2011) Translocator protein PET imaging for glial activation in multiple sclerosis. J Neuroimmune Pharmacol 6(3):354–361. https://doi.org/10.1007/s11481-010-9243-6
Rissanen E, Tuisku J, Rokka J, Paavilainen T, Parkkola R, Rinne JO, Airas L (2014) In vivo detection of diffuse inflammation in secondary progressive multiple sclerosis using PET imaging and the radioligand 11C-PK11195. J Nucl Med 55(6):939–944. https://doi.org/10.2967/jnumed.113.131698
Giannetti P, Politis M, Su P, Turkheimer FE, Malik O, Keihaninejad S, Wu K, Waldman A, Reynolds R, Nicholas R, Piccini P (2015) Increased PK11195-PET binding in normal-appearing white matter in clinically isolated syndrome. Brain 138(Pt. 1):110–119. https://doi.org/10.1093/brain/awu331
Herranz E, Giannì C, Louapre C, Treaba CA, Govindarajan ST, Ouellette R, Loggia ML, Sloane JA, Madigan N, Izquierdo-Garcia D, Ward N, Mangeat G, Granberg T, Klawiter EC, Catana C, Hooker JM, Taylor N, Ionete C, Kinkel RP, Mainero C (2016) Neuroinflammatory component of gray matter pathology in multiple sclerosis. Ann Neurol 80(5):776–790. https://doi.org/10.1002/ana.24791
Barletta VT, Herranz E, Treaba CA, Ouellette R, Mehndiratta A, Loggia ML, Klawiter EC, Ionete C, Sloane JA, Mainero C (2020) Evidence of diffuse cerebellar neuroinflammation in multiple sclerosis by 11 C-PBR28 MR-PET. Mult Scler 26(6):668–678. https://doi.org/10.1177/1352458519843048
Sucksdorff M, Matilainen M, Tuisku J, Polvinen E, Vuorimaa A, Rokka J, Nylund M, Rissanen E, Airas L (2020) Brain TSPO-PET predicts later disease progression independent of relapses in multiple sclerosis. Brain 143(11):3318–3330. https://doi.org/10.1093/brain/awaa275
Datta G, Colasanti A, Kalk N, Owen D, Scott G, Rabiner EA, Gunn RN, Lingford-Hughes A, Malik O, Ciccarelli O, Nicholas R, Nei L, Battaglini M, De Stefano N, Matthews PM (2017) 11C-PBR28 and 18F-PBR111 detect white matter inflammatory heterogeneity in multiple sclerosis. J Nucl Med 58(9):1477–1482. https://doi.org/10.2967/jnumed.116.187161
Nutma E, Stephenson JA, Gorter RP, de Bruin J, Boucherie DM, Donat CK, Breur M, van der Valk P, Matthews PM, Owen DR, Amor S (2019) A quantitative neuropathological assessment of translocator protein expression in multiple sclerosis. Brain 142(11):3440–3455. https://doi.org/10.1093/brain/awz287
Herranz E, Louapre C, Treaba CA, Govindarajan ST, Ouellette R, Mangeat G, Loggia ML, Cohen-Adad J, Klawiter EC, Sloane JA, Mainero C (2020) Profiles of cortical inflammation in multiple sclerosis by 11C-PBR28 MR-PET and 7 Tesla imaging. Mult Scler 26(12):1497–1509. https://doi.org/10.1177/1352458519867320
Warntjes M, Engström M, Tisell A, Lundberg P (2016) Modelling the presence of myelin and oedema in the brain based on multi-parametric quantitative MRI. Front Neurol 7:16. https://doi.org/10.3389/fneur.2016.00016
Granberg T, Fan Q, Treaba CA, Ouellette R, Herranz E, Gabriel Mangeat G, Louapre C, Cohen-Adad J, Klawiter EC, Sloane JA, Mainero C (2017) In vivo characterization of cortical and white matter neuroaxonal pathology in early multiple sclerosis. Brain 140(11):2912–2926. https://doi.org/10.1093/brain/awx247
Ouellette R, Mangeat G, Polyak I, Warntjes M, Forslin Y, Bergendal Å, Plattén M, Uppman M, Treaba CA, Cohen-Adad J, Piehl F, Kristoffersen Wiberg M, Fredrikson S, Mainero C, Granberg T (2020) Validation of rapid magnetic resonance myelin imaging in multiple sclerosis. Ann Neurol 87(5):710–724. https://doi.org/10.1002/ana.25705
Owen DR, Yeo AJ, Gunn RN, Song K, Wadsworth G, Lewis A, Rhodes C, Pulford DJ, Bennacef I, Parker CA, StJean PL, Cardon LR, Mooser VE, Matthews PM, Rabiner EA, Rubio JP (2012) An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab 32(1):1–5. https://doi.org/10.1038/jcbfm.2011.147
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O’Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69(2):292–302. https://doi.org/10.1002/ana.22366
Parmenter BA, Testa SM, Schretlen DJ, Weinstock-Guttman B, Benedictet RHB (2010) The utility of regression-based norms in interpreting the minimal assessment of cognitive function in multiple sclerosis (MACFIMS). J Int Neuropsychol Soc 16(1):6–16. https://doi.org/10.1017/S1355617709990750
Stankoff B, Poirion E, Tonietto M, Bodini B (2018) Exploring the heterogeneity of MS lesions using positron emission tomography: a reappraisal of their contribution to disability. Brain Pathol 28(5):723–734. https://doi.org/10.1111/bpa.12641
Moccia M, van de Pavert S, Eshaghi A, Haider L, Pichat J, Yiannakas M, Ourselin S, Wang Y, Wheeler-Kingshott C, Thompson A, Barkhof F, Ciccarelli O (2020) Pathologic correlates of the magnetization transfer ratio in multiple sclerosis. Neurology 95(22):e2965–e2976. https://doi.org/10.1212/WNL.0000000000010909
Warntjes M, Engström M, Tisell A, Lundberg P (2016) Modeling the presence of myelin and edema in the brain based on multi-parametric quantitative MRI. Front Neurol 7:16. https://doi.org/10.3389/fneur.2016.00016
Giannetti P, Politis M, Su P, Turkheimer F, Malik O, Keihaninejad S, Wu K, Reynolds R, Nicholas R, Piccini P (2014) Microglia activation in multiple sclerosis black holes predicts outcome in progressive patients: an in vivo [(11)C](R)-PK11195-PET pilot study. Neurobiol Dis 65:203–210. https://doi.org/10.1016/j.nbd.2014.01.018
Absinta M, Sati P, Masuzzo F, Nair G, Sethi V, Kolb H, Ohayon J, Wu T, Cortese ICM, Reich DS (2019) Association of chronic active multiple sclerosis lesions with disability in vivo. JAMA Neurol 76(12):1474–1483. https://doi.org/10.1001/jamaneurol.2019.2399
Prineas JW, Kwon EE, Cho ES, Sharer LR (1984) Continual breakdown and regeneration of myelin in progressive multiple sclerosis plaques. Ann N Y Acad Sci 436:11–32. https://doi.org/10.1111/j.1749-6632.1984.tb14773.x
Frischer JM, Weigand SD, Guo Y, Kale N, Parisi JE, Pirko I, Mandrekar J, Bramow S, Metz I, Brück W, Lassmann H, Lucchinetti CF (2015) Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann Neurol 78(5):710–721. https://doi.org/10.1002/ana.24497
Kuhlmann T, Ludwin S, Prat A, Antel J, Brück W, Lassmann H (2017) An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol 133(1):13–24. https://doi.org/10.1007/s00401-016-1653-y
Hughes AN, Appel B (2020) Microglia phagocytose myelin sheaths to modify developmental myelination. Nat Neurosci 23(9):1055–1066. https://doi.org/10.1038/s41593-020-0654-2
Kopper TJ, Gensel JC (2018) Myelin as an inflammatory mediator: Myelin interactions with complement, macrophages, and microglia in spinal cord injury. J Neurosci Res 96(6):969–977. https://doi.org/10.1002/jnr.24114
Lombardi M, Paroloisi R, Scaroni F, Bonfanti E, Gualerzi A, Gabrielli M, Kerlero de Rosbo N, Uccelli A, Giussani P, Viani P, Garlanda C, Abbracchio MP, Chaabane L, Buffo A, Fumagalli M, Verderio C (2019) Detrimental and protective action of microglial extracellular vesicles on myelin lesions: astrocyte involvement in remyelination failure. Acta Neuropathol 138(6):987–1012. https://doi.org/10.1007/s00401-019-02049-1
Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, van Wijngaarden P, Wagers AJ, Williams A, Franklin RJM, Ffrench-Constant C (2013) M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci 16(9):1211–1218. https://doi.org/10.1038/nn.3469
Albert M, Antel J, Brück W, Stadelmann C (2007) Extensive cortical remyelination in patients with chronic multiple sclerosis. Brain Pathol 17(2):129–138. https://doi.org/10.1111/j.1750-3639.2006.00043.x
Strijbis EMM, Kooi EJ, van der Valk P, Geurts JJG (2017) Cortical remyelination is heterogeneous in multiple sclerosis. J Neuropathol Exp Neurol 76(5):390–401. https://doi.org/10.1093/jnen/nlx023
Bodini B, Veronese M, García-Lorenzo D, Battaglini M, Poirion E, Chardain A, Freeman L, Louapre C, Tchikviladze M, Papeix C, Dollé F, Zalc B, Lubetzki C, Bottlaender M, Turkheimer F, Stankoff B (2016) Dynamic imaging of individual remyelination profiles in multiple sclerosis. Ann Neurol 79(5):726–738. https://doi.org/10.1002/ana.24620
Clarke MA, Lakhani DA, Wen S, Gao S, Smith SA, Dortch R, Xu J, Bagnato F (2021) Perilesional neurodegenerative injury in multiple sclerosis: Relation to focal lesions and impact on disability. Mult Scler Relat Disord. 49:102738. https://doi.org/10.1016/j.msard.2021.102738
Lampron A, Larochelle A, Laflamme N, Préfontaine P, Plante M, Sánchez MG, Yong VW, Stys PK, Tremblay M, Rivest S (2015) Inefficient clearance of myelin debris by microglia impairs remyelinating processes. J Exp Med 212(4):481–495. https://doi.org/10.1084/jem.20141656
Safaiyan S, Kannaiyan N, Snaidero N, Brioschi S, Biber K, Yona S, Edinger AL, Jung S, Rossner MJ, Simons M et al (2016) Age-related myelin degradation burdens the clearance function of microglia during aging. Nat Neurosci 19(8):995–998. https://doi.org/10.1038/nn.4325
Zivadinov R, Leist TP (2005) Clinical-magnetic resonance imaging correlations in multiple sclerosis. J Neuroimaging 15(4 Suppl):10S-21S. https://doi.org/10.1177/1051228405283291
Rissanen E, Tuisku J, Vahlberg T, Sucksdorff M, Paavilainen T, Parkkola R, Rokka J, Gerhard A, Hinz R, Talbot PS, Rinne JO, Airas L (2018) Microglial activation, white matter tract damage, and disability in MS. Neurol Neuroimmunol Neuroinflamm. 5(3):e443. https://doi.org/10.1212/NXI.0000000000000443
Funding
This study was supported by the National Multiple Sclerosis Society (NMSS RG-1802-30468), NIH 1R21NS123419-01 and by Sanofi Genzyme.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Carolina Ionete has received research support from Biogen, Genentech, and consulting compensation from Genzyme. The other Authors declare no conflicts of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Barletta, V., Herranz, E., Treaba, C.A. et al. In vivo characterization of microglia and myelin relation in multiple sclerosis by combined 11C-PBR28 PET and synthetic MRI. J Neurol 270, 3091–3102 (2023). https://doi.org/10.1007/s00415-023-11621-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-11621-5