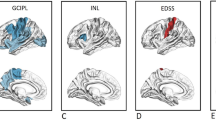
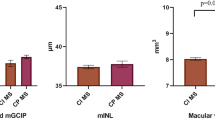
Abstract
Background
Several studies report mixed associations between the retinal nerve fiber layer (RNFL) thickness with cognitive and physical disability in persons with multiple sclerosis (PwMS). Systematic synthesis of these findings is crucial in deriving credible conclusions.
Methods
Five databases were searched from their inception to March 2022. The inclusion criteria for studies were MS-specific and required RNFL and cognitive performance data in order to be analyzed. The selection processes followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Results
The systematic review yielded 31 studies that investigated the association between RNFL thickness and cognitive performance. Twenty-two studies reported positive associations, and nine did not. The meta-analysis included 11 studies with a total of 782 PwMS with mean age of 40.5 years, mean Expanded Disability Status Scale (EDSS) of 2.7, and disease duration of 11.3 years. RNFL thickness was significantly associated Symbol Digit Modalities Test (pooled r = 0.306, p < 0.001), Paced Auditory Serial Addition Test (pooled r = 0.374, p < 0.001) and Word List Generation (WLG, pooled r = 0.177, p < 0.001). RNFL was also significantly correlated with visuospatial learning and memory tests (pooled r = 0.148, p = 0.042) and verbal learning and memory tests (pooled r = 0.245, p = 0.005). Within three eligible studies, no significant association between ganglion cell inner-plexiform layer and SDMT 0.083 (95% CI − 0.186, 0.352) was noted. The heterogeneity was high in all correlation studies (I2 > 63% and p < 0.008) except for the WLG and visuospatial memory findings.
Conclusion
RNFL thickness is associated with cognitive processing speed, verbal learning and memory, visual learning and memory, as well as verbal fluency in PwMS. The number of studies included in the meta-analyses were limited due to non-standardized reporting.






Similar content being viewed by others
References
Lassmann H (2014) Mechanisms of white matter damage in multiple sclerosis. Glia 62(11):1816–1830
MacAllister WS et al (2005) Cognitive functioning in children and adolescents with multiple sclerosis. Neurology 64(8):1422–1425
Kutzelnigg A, Lassmann H (2005) Cortical lesions and brain atrophy in MS. J Neurol Sci 233(1–2):55–59
Stadelmann C, Wegner C, Brück W (2011) Inflammation, demyelination, and degeneration—recent insights from MS pathology. Biochimica et Biophysica Acta (BBA)-Mol Basis Dis 1812(2):275–282
Harvey PD (2019) Domains of cognition and their assessment. Dialogues Clin Neurosci 21(3):227–237
Tan HEI et al (2018) Optical coherence tomography of the tympanic membrane and middle ear: a review. Otolaryngol-Head Neck Surgery 159(3):424–438
Chiaravalloti ND, DeLuca J (2008) Cognitive impairment in multiple sclerosis. Lancet Neurol 7(12):1139–1151
Gupta S et al (2016) Optical coherence tomography and neurodegeneration: are eyes the windows to the brain? Expert Rev Neurother 16(7):765–775
Baetge SJ et al (2021) Association of retinal layer thickness with cognition in patients with multiple sclerosis. Neurol Neuroimmunol Neuroinflammation 8(4):e1018
Esmael A et al (2020) Retinal thickness as a potential biomarker of neurodegeneration and a predictor of early cognitive impairment in patients with multiple sclerosis. Neurol Res 42(7):564–574
Coric D et al (2018) Cognitive impairment in patients with multiple sclerosis is associated with atrophy of the inner retinal layers. Mult Scler J 24(2):158–166
Ashtari F, Emami P, Akbari M (2015) Association between retinal nerve fiber layer thickness and magnetic resonance imaging findings and intelligence in patients with multiple sclerosis. Adv Biomed Res 4:223
Coric D et al (2018) Cognitive impairment in patients with multiple sclerosis is associated with atrophy of the inner retinal layers. Mult Scler 24(2):158–166
Dreyer-Alster S et al (2022) Optical coherence tomography is associated with cognitive impairment in multiple sclerosis. J Neuroophthalmol 42(1):e14–e21
Uzunköprü C et al (2021) Retinal nerve fiber layer thickness correlates with serum and cerebrospinal fluid neurofilament levels and is associated with current disability in multiple sclerosis. Noropsikiyatri Arsivi 58(1):34–40
Gencer M et al (2011) Retinal nerve fibre layer thinning is associated with physical and cognitive disability, and disease severity. Mult Scler 17(10):S89–S89
Giedraitiene N et al. (2021) Table_1_Cognitive decline in multiple sclerosis is related to the progression of retinal atrophy and presence of oligoclonal bands: a 5-year follow-up study. XLSX
Frau J et al (2018) A cross-sectional and longitudinal study evaluating brain volumes, RNFL, and cognitive functions in MS patients and healthy controls. BMC Neurol 18(1):67
Pul R et al (2016) Longitudinal time-domain optic coherence study of retinal nerve fiber layer in IFNβ-treated and untreated multiple sclerosis patients. Exp Ther Med 12(1):190–200
Wieder L et al (2013) Low contrast visual acuity testing is associated with cognitive performance in multiple sclerosis: a cross-sectional pilot study. BMC Neurol 13:1–8
Talmage GD et al (2017) Natalizumab stabilizes physical, cognitive, MRI, and OCT markers of disease activity: a prospective, non-randomized pilotstudy. PLoS ONE 12(4):e0173299
Stroup DF et al (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 283(15):2008–2012
Thompson AJ et al (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 17(2):162–173
Riccardi A et al (2019) D-KEFS ST failure identifies multiple sclerosis patients with worse objective and self-perceived physical and cognitive disability. Front Psychol 10:49–49
Sabry HM, Ibrahim MA (2010) Optical coherence tomography of retinal nerve fibre layer in multiple sclerosis and its correlation with the disease evolution. Egypt J Neurol, Psychiatry Neurosurgery 47(1):21–27
Silva R et al (2022) Cognitive impairment and markers of optical neurodegeneration in early multiple sclerosis. Neurol Sci 43:4381–4386
El Ayoubi NK et al (2021) Effect of fingolimod vs interferon treatment on OCT measurements and cognitive function in RRMS. Mult Scler Relat Disord 53:103041
Naseer MA et al (2020) Early detection of cognitive dysfunction in patients with multiple sclerosis: implications on outcome. Brain Impairment 21(2):208–216
Stellmann JP et al (2017) Pattern of gray matter volumes related to retinal thickness and its association with cognitive function in relapsing–remitting MS. Brain Behav 7(2):e00614
Peterson J et al (2011) The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hosp Res Inst 2(1):1–12
Hardy RJ, Thompson SG (1998) Detecting and describing heterogeneity in meta-analysis. Stat Med 17(8):841–856
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Moher D et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151(4):264–269
Parums DV (2021) Review articles, systematic reviews, meta-analysis, and the updated preferred reporting items for systematic reviews and meta-analyses (PRISMA) 2020 guidelines. Med Sci Monitor: Int Med J Exp Clin Res 27:e934475–e934481
Abdel Naseer M et al (2019) Cognitive and physical disability in Egyptian patients with multiple sclerosis: genetic and optical coherence tomography study. Neurol Res 41(7):644–651
Bsteh G et al (2021) Macular ganglion cell–inner plexiform layer thinning as a biomarker of disability progression in relapsing multiple sclerosis. Mult Scler J 27(5):684–694
El Ayoubi NK et al (2016) Retinal measures correlate with cognitive and physical disability in early multiple sclerosis. J Neurol 263(11):2287–2295
Toledo J et al (2008) Retinal nerve fiber layer atrophy is associated with physical and cognitive disability in multiple sclerosis. Mult Scler 14(7):906–912
Birkeldh U et al (2019) Retinal nerve fiber layer thickness associates with cognitive impairment and physical disability in multiple sclerosis. Mult Scler Relat Disord 36:101414
Birkeldh U et al (2017) The temporal retinal nerve fiber layer thickness is the most important optical coherence tomography estimate in multiple sclerosis. Front Neurol 8:675
Jakimovski D et al (2021) Visual deficits and cognitive assessment of multiple sclerosis: confounder, correlate, or both? J Neurol 268(7):2578–2588
Nguyen J et al (2018) Visual pathway measures are associated with neuropsychological function in multiple sclerosis. Curr Eye Res 43(7):941–948
Petracca M et al (2017) Looking into cognitive impairment in primary-progressive MS. Neurol 88(16):192–195
Bsteh G et al (2020) Retinal layer thinning is reflecting disability progression independent of relapse activity in multiple sclerosis. Mult Scler J Exp Transl Clin 6(4):2055217320966344
Bsteh G et al (2019) Peripapillary retinal nerve fibre layer thinning rate as a biomarker discriminating stable and progressing relapsing–remitting multiple sclerosis. Eur J Neurol 26(6):865–871
Bsteh G et al (2019) Peripapillary retinal nerve fibre layer as measured by optical coherence tomography is a prognostic biomarker not only for physical but also for cognitive disability progression in multiple sclerosis. Mult Scler J 25(2):196–203
Collorone S et al (2022) Visual function and brief cognitive assessment for multiple sclerosis in optic neuritis clinically isolated syndrome patients. J Neuroophthalmol 42(1):e22–e31
Bsteh G et al (2021) Retinal layer thinning predicts treatment failure in relapsing multiple sclerosis. Eur J Neurol 28(6):2037–2045
Benedict RHB et al (2012) Brief international cognitive assessment for MS (BICAMS): international standards for validation. BMC Neurol 12(1):1–7
Sepulcre J et al (2006) Cognitive impairment in patients with multiple sclerosis using the brief repeatable battery-neuropsychology test. Mult Scler J 12(2):187–195
Benedict RHB et al (2006) Validity of the minimal assessment of cognitive function in multiple sclerosis (MACFIMS). J Int Neuropsychol Soc 12(4):549–558
Klein OA et al (2019) Assessment and management of cognitive problems in people with multiple sclerosis: a national survey of clinical practice. Int J Clin Pract 73(3):e13300–e13300
Connick P, Chandran S, Bak TH (2013) Patterns of cognitive dysfunction in progressive MS. Behav Neurol 27(3):259–265
Sonder JM et al (2014) Comparing long-term results of PASAT and SDMT scores in relation to neuropsychological testing in multiple sclerosis. Mult Scler J 20(4):481–488
Rao SM et al (2014) Correlations between MRI and information processing speed in MS: a meta-analysis. Mult Scler Int 2014:975803
Doustar J et al (2017) Optical coherence tomography in Alzheimer’s disease and other neurodegenerative diseases. Front Neurol 8:701
Coppola G et al (2015) Optical coherence tomography in Alzheimer’s disease: a meta-analysis. PLoS ONE 10(8):e0134750–e0134750
Ko F et al (2018) Association of retinal nerve fiber layer thinning with current and future cognitive decline: a study using optical coherence tomography. JAMA Neurol 75(10):1198–1205
Knoll B et al (2016) Retinal nerve fiber layer thickness in amnestic mild cognitive impairment: case-control study and meta-analysis. Alzheimers Dement (Amst) 4:85–93
Shen Y et al (2014) Retinal nerve fiber layer thickness is associated with episodic memory deficit in mild cognitive impairment patients. Curr Alzheimer Res 11(3):259–266
Dragoi V, Tsuchitani C. Chapter 15: visual processing: cortical pathways. Neuroscience Online, UTHealth, vol 29. Accessed Jun 2016
Bergsland N et al (2016) Localized atrophy of the thalamus and slowed cognitive processing speed in MS patients. Mult Scler 22(10):1327–1336
Bergsland N et al (2021) Thalamic nuclei volumes and their relationships to neuroperformance in multiple sclerosis: A cross-sectional structural MRI study. J Magn Reson Imaging 53(3):731–739
Batista S et al (2012) Basal ganglia, thalamus and neocortical atrophy predicting slowed cognitive processing in multiple sclerosis. J Neurol 259(1):139–146
Berman RA, Wurtz RH (2008) Exploring the pulvinar path to visual cortex. Prog Brain Res 171:467–473
Casanova C et al (2001) Higher-order motion processing in the pulvinar. Prog Brain Res 134:71–82
McAlonan K, Cavanaugh J, Wurtz RH (2008) Guarding the gateway to cortex with attention in visual thalamus. Nature 456(7220):391–394
Pawlitzki M et al (2020) MS optic neuritis-induced long-term structural changes within the visual pathway. Neurol Neuroimmunol Neuroinflamm 7(2):e665
Puthenparampil M et al (2017) Trans-synaptic degeneration in the optic pathway: a study in clinically isolated syndrome and early relapsing-remitting multiple sclerosis with or without optic neuritis. PLoS ONE 12(8):e0183957
Zivadinov R et al (2014) Retinal nerve fiber layer thickness and thalamus pathology in multiple sclerosis patients. Eur J Neurol 21(8):1137-e61
Frohman EM et al (2009) Relationship of optic nerve and brain conventional and non-conventional MRI measures and retinal nerve fiber layer thickness, as assessed by OCT and GDx: a pilot study. J Neurol Sci 282(1–2):96–105
Vural A et al (2020) Retinal degeneration is associated with brain volume reduction and prognosis in radiologically isolated syndrome. Mult Scler 26(1):38–47
Pietroboni AM et al (2020) Evidence of retinal anterograde neurodegeneration in the very early stages of multiple sclerosis: a longitudinal OCT study. Neurol Sci 41(11):3175–3183
Glasner P et al (2022) Retinal nerve fiber and ganglion cell complex layer thicknesses mirror brain atrophy in patients with relapsing-remitting multiple sclerosis. Restor Neurol Neurosci 40(1):35–42
Jakimovski D et al (2019) Cognitive profiles of aging in multiple sclerosis. Front Aging Neurosci 11:105
Gromisch ES, Foley FW (2020) Can semantic fluency be used as an alternative or additional measure in the abbreviated minimal assessment of cognitive function in multiple sclerosis (aMACFIMS)? J Neurol Sci 410:116640
Jakimovski D et al (2021) Clinical effects associated with five-year retinal nerve fiber layer thinning in multiple sclerosis. J Neurol Sci 427:117552
Seo S et al (2017) Ganglion cell-inner plexiform layer and retinal nerve fiber layer thickness according to myopia and optic disc area: a quantitative and three-dimensional analysis. BMC Ophthalmol 17(1):1–8
Saidha S, Calabresi PA (2014) Optical coherence tomography should be part of the routine monitoring of patients with multiple sclerosis: yes. Mult Scler 20(10):1296–1298
Saidha S et al (2011) Visual dysfunction in multiple sclerosis correlates better with optical coherence tomography derived estimates of macular ganglion cell layer thickness than peripapillary retinal nerve fiber layer thickness. Mult Scler J 17(12):1449–1463
Syc SB et al (2012) Optical coherence tomography segmentation reveals ganglion cell layer pathology after optic neuritis. Brain 135(2):521–533
Ratchford JN et al (2013) Active MS is associated with accelerated retinal ganglion cell/inner plexiform layer thinning. Neurology 80(1):47–54
Oertel FC et al (2018) Retinal ganglion cell loss in neuromyelitis optica: a longitudinal study. J Neurol Neurosurg Psychiatry 89(12):1259–1265
Balk LJ et al (2016) Timing of retinal neuronal and axonal loss in MS: a longitudinal OCT study. J Neurol 263(7):1323–31
Aytulun A et al (2021) APOSTEL 2.0 Recommendations for Reporting Quantitative Optical Coherence Tomography Studies. Neurology 2021:97(2):68–79. https://doi.org/10.1212/WNL.0000000000012125
Acknowledgements
The authors want to specifically acknowledge Elham Moases Ghaffary and Alireza Afshari-Safavi for their contribution in data collection and analysis of the article.
Funding
We did not receive any financial support for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest regarding the publication of this paper. Omid Mirmosayyeb has nothing to disclose. Robert Zivadinov has received personal compensation from Bristol Myers Squibb, EMD Serono, Sanofi, Protembis, Janssen, 415 Capital, and Novartis for speaking and consultant fees. He received financial support for research activities from Sanofi, Novartis, Bristol Myers Squibb, Octave, Mapi Pharma, CorEvitas, Protembis and V-WAVE Medical. Bianca Weinstock-Guttman received honoraria as a speaker and/or as a consultant for Biogen Idec, Teva Pharmaceuticals, EMD Serono, Genzyme, Sanofi, Genentech, Novartis, Celgene/BMS, Janssen and Horizon Dr Weinstock-Guttman received research funds from Biogen Idec, EMD Serono, Genzyme, Genentech, Sanofi, Novartis. Ralph HB. Benedict has received consultation or speaking fees from Bristol Myer Squibb, Biogen, Merck, EMD Serono, Roche, Verasci, Immune Therapeutics, Novartis, and Sanofi-Genzyme. Dejan Jakimovski serves as Associate Editor of Clinical Neurology and Neurosurgery and compensated by Elsevier B.V.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mirmosayyeb, O., Zivadinov, R., Weinstock-Guttman, B. et al. Optical coherence tomography (OCT) measurements and cognitive performance in multiple sclerosis: a systematic review and meta-analysis. J Neurol 270, 1266–1285 (2023). https://doi.org/10.1007/s00415-022-11449-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-022-11449-5