Abstract
Background/Objective
Reversible cerebral vasoconstriction syndrome may be underdiagnosed. It can be accompanied by various complications, mainly intracerebral hemorrhage and ischemic stroke. The clinical presentation of this condition varies according to its localization. The aims of this review are to raise awareness of the disease, especially in the presence of corresponding risk factors; to connect its precipitating factors, pathophysiology, and complications; and to compare various differential diagnoses of vasoconstriction.
Methods
A review of the literature in PubMed/MEDLINE and Google Scholar was conducted from May 1997 until May 2022.
Results
Reversible cerebral vasoconstriction syndrome, which is a clinical–radiological syndrome, is mainly characterized by the occurrence of thunderclap headache and widespread vasoconstriction. The most common precipitating factors are the use of vasoactive substances and postpartum status. The pathophysiology is currently assumed to include two mechanisms: sympathetic overactivity and endothelial dysfunction. From these mechanisms, it is possible to derive potential complications as well as the most important differential diagnoses: posterior reversible encephalopathy syndrome, convexity subarachnoid hemorrhage, ischemic and hemorrhagic stroke, and primary angiitis of the central nervous system.
Conclusion
In general, the outcome of reversible cerebral vasoconstriction syndrome is very good. Vasospasm as well as thunderclap headache attacks can be fully reversible, and > 90% of patients are functionally independent at discharge.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reversible cerebral vasoconstriction syndrome (RCVS), whose cardinal symptom is acute, worst-ever headache (thunderclap headache (TCH)) in approximately 85% of cases, is mainly a clinical–radiological syndrome and may or may not include neurological deficits [1,2,3]. The clinical presentation often depends on complications such as ischemic stroke, intracranial hemorrhage, and posterior reversible encephalopathy syndrome. Depending on the affected area, aphasia, hemiparesis, hemianopsia, epileptic seizures, or Balint syndrome may occur in up to 43% of cases [4]. In > 85% of cases, a characteristic episode of TCH can be seen approximately 2–3 weeks prior [5]. Cranial imaging by computed tomography (CT) or magnetic resonance imaging (MRI) should be followed by vascular imaging with CT angiography or MR angiography [6]. Aside from the treatment of acute complications, such as stroke, intracranial hemorrhage, and epileptic seizures, the first-line drug for RCVS treatment is the calcium channel antagonist nimodipine [7,8,9]. It is administered first orally and then intra-arterially in fulminant cases [9, 10]. In 2007, Calabrese et al. characterized RCVS, for which there already existed multiple case reports and different terms (Table 1) [6, 11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28]. A detailed overview of RCVS cases characterized by risk factors/triggers and complications can be found in the “Supplementary Information” section (see Online Reference 1). Diagnostic criteria have also been established (Table 2) [11]. RCVS is mainly marked by widespread vasoconstriction from distal to proximal vascular segments, which regresses within 3 months and is mainly precipitated by various factors, such as vasoactive drug intake or postpartum status [11]. The pathophysiology of this syndrome is still not fully understood [29], but it is thought to encompass two main mechanisms: endothelial dysfunction and disturbances in cerebral vascular tone caused primarily by sympathetic overactivation [6, 12, 30,31,32,33,34,35,36].
The aim of this narrative review is to link the precipitating factors, pathophysiology and complications of RCVS. Another important point is the detailed analysis of possible differential diagnoses to prevent misdiagnosis and protect patients from unnecessary, risky diagnostics, and even harmful therapy.
Methods
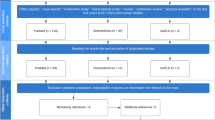
The systematic literature search for this review encompassed the PubMed/MEDLINE and Google Scholar databases, and was performed by the first author (DKE). The search terms (in PubMed/MEDLINE) were "RCVS pathophysiology", “RCVS AND serotonergic drugs”, “RCVS and corticosteroids”, “RCVS AND thunderclap headache”, “RCVS AND postpartum”, "RCVS AND COVID-19", "RCVS AND stroke", “RCVS AND intracranial hemorrhage”, "RCVS AND PRES" (posterior reversible encephalopathy syndrome), "RCVS AND PACNS" (primary angiitis of the central nervous system), “RCVS AND treatment”, and “RCVS AND imaging”; articles published from May 1997 to May 2022 were considered. In addition, the search was extended to the bibliographies of the articles found with the terms mentioned above. PubMed/MEDLINE and Google Scholar were used for this purpose. Only articles published in English or German were included. The results of the literature search with the above-mentioned terms is shown in Table 3, and a flowchart of the literature search is shown in Fig. 1.
The literature search is illustrated in this flowchart. The literature search described under "Methods" identified 1154 articles that could potentially be considered. In a two-stage selection process using the exclusion criteria mentioned above, 118 articles were ultimately included in the review
Results
Epidemiology
The incidence of RCVS is unknown but steadily increasing. In general, RCVS can affect any age group, even adolescents and children, but the mean age is between 42 and 47 years [4, 37,38,39]. Depending on the study, the gender ratio varies from 1.8:1 to 8:1, with a female predominance [4, 6]. Explanations for the variability of the ratio may include the involvement of different diagnostic criteria and/or differences in recruiting centers (emergency room vs. headache clinic; outpatient vs. inpatient) [40, 41]. The ratio for secondary RCVS (caused by a risk factor) is slightly reduced [6]. On average, male patients are a decade younger than female patients (third-to-fourth decade vs. fourth-to-fifth decade, respectively) [42]. Surprisingly, in children/adolescents, up to 85% of patients are male [43]. Reasons for the discrepancy with adults have not yet been identified. Generally, in > 90% of cases, a risk factor has been identified [43,44,45].
Risk factors and triggers
RCVS can occur spontaneously/idiopathically, without a precipitating factor; in other patients, it occurs secondarily (approximately 40–60% of cases) [1, 46]. The precipitating factors and triggers of RCVS and TCH, respectively, are manifold (Table 4), which illustrates why the exact pathophysiology is not yet fully understood [11, 12, 17, 47,48,49,50,51,52,53,54,55,56,57,58,59,60,61]. Trigger factors for the most severe headache in a patient’s life can include coughing, bathing, physical exertion, Valsalva maneuvers, and sexual activity [55, 62,63,64,65]. Headaches caused by trigger factors may represent a primary headache disorder [66]. However, when present in combination with focal neurological deficits and appropriate angiographic findings, these factors may be considered etiologically to be triggers of RCVS [19, 20, 55, 62,63,64]. According to two of the four main studies, the most frequent precipitating factors, accounting for 31% of cases overall, are vasoactive medications (especially serotonergic drugs) and the postpartum period [6, 67]. Vasoactive substances mainly include serotonergic drugs, such as selective serotonin reuptake inhibitors (SSRIs); serotonin–noradrenaline reuptake inhibitors (SNRIs); 3,4-methylenedioxymethamphetamine (ecstasy); and triptans and sympathomimetics with serotonergic effects, such as over-the-counter drugs for upper respiratory tract infections (e.g., pseudoephedrine), amphetamines, cocaine, and ergot derivatives [13, 14, 49, 50, 68,69,70]. The use of these substances in combination with each other or with cannabis or opioids (which also have known SSRI effects) has already led to fatal cases of RCVS [46, 53, 71,72,73]. Vasoactive drugs act via specific receptors (antidepressants: 5-HT1B and 5-HT2A; triptans: 5-HT1B and 5-HT1D) located on smooth muscle cells in the area of peripheral and central blood vessels, and serotonergic–sympathomimetic synergism can cause potent cerebral/myocardial vasoconstriction [4, 13, 14, 47]. An important precipitating factor for men is cannabis use (up to 32% in the French case series), whose ingredient tetrahydrocannabinol can trigger vasoconstriction in different models [46, 71, 72]. In up to 40% of RCVS cases, there is a history of migraine [4, 6, 8]. One reason may be that triptans are precipitating factors for RCVS [50].
RCVS in the postpartum period, previously known as postpartum angiopathy (PPA), usually peaks 2 weeks after delivery [42, 48]. Many case reports and smaller case series have reported the coexistence of widespread cerebral vasoconstriction and pronounced parieto-occipital vasogenic edema, also referred to as posterior reversible encephalopathy syndrome (PRES), in the postpartum state [12, 15, 35, 47, 74]. The presence of eclampsia is a major risk factor for the development of PRES (24–47%) [15]. In addition, there is also a close association of PRES with RCVS as a complication in 7–38% of cases [4, 6, 72]. According to the American College of Obstetricians and Gynecologists (ACOG), eclampsia is defined as pre-eclampsia with the occurrence of new seizures or coma [75]. In 25% of cases, it occurs in the postpartum period [47]. With acute severe headache or TCH, seizures, hypertension, and focal neurological deficits, RCVS and eclampsia share several common symptoms, mostly neurological [12, 35, 76,77,78,79]. Moreover, in more than half of RCVS patients postpartum, characteristic manifestations of eclampsia, e.g., hypertension and PRES, are observed [15]. With regard to clinical (seizures, hemiparesis, and visual deficits) and radiological (vasoconstriction, restricted diffusion, PRES) features, RCVS/PPA and eclampsia have some overlap [12, 15, 74]. One article also speaks of distinct spectra of a disease process with the same fundamental pathophysiology, namely, endothelial dysfunction, as will be discussed in more detail in the next section [15].
In summary, the previous explanations help us to understand why RCVS has such a female predominance [1, 4]. According to Topcuoglu et al., nonpregnant women with RCVS were, on average, older than male patients (48 ± 11 years vs. 34 ± 13 years) and had more underlying depression, antidepressant use, and migraine [42]. In the following, the underlying pathophysiology will be explained in more detail, with emphasis on hormonal differences between the sexes and their role in vasoconstriction.
Excursion: RCVS and COVID-19
A limited number of case reports and one small case series of RCVS related to COVID-19, including mild respiratory infection, have been published [52, 80,81,82]. In 30% of COVID-19 patients with RCVS, none of the previously known precipitants could be detected [80]. Enhanced activation of the classical renin–angiotensin system (RAS) axis with sympathetic overactivation, which may lead to vasoconstriction of cerebral vessels, was postulated to occur via downregulation of the angiotensin-converting enzyme 2 (ACE2) receptor directly by SARS-CoV-2 [52, 81, 83]. This mechanism, as well as a coagulopathic/proinflammatory state in the context of critical illness, is also known to lead to ischemia in the context of COVID-19 [84]. Treatment with nimodipine was successful in most cases [81]. The severity of COVID-19 infection was not indicative of the extent of RCVS and its potential complications [80, 81].
Clinical presentation
First, it must be noted that in 85–100% of cases, RCVS is associated with recurrent episodes of TCH, that is, worst-ever headache that peaks in ≤ 1 min and is often accompanied by screaming and crying (32%), photophobia (30%), nausea (57%), and vomiting (38%) [5, 6, 38]. Characteristically, these episodes occur in clusters within up to 4 weeks and may or may not be accompanied by focal neurological deficits and/or epileptic seizures, as noted in the beta version of the International Classification of Headache Disorders 3 (ICHD-3) criteria [66]. In up to one-third of all patients, hypertensive blood pressure levels occur during the attacks [6]. In the time between TCH attacks, a slight, persistent headache may occur in approximately 35% of cases [6]. TCH is not pathognomonic for RCVS, because it may also be the first sign of other conditions: subarachnoid hemorrhage (SAH) due to ruptured aneurysm, cerebral sinus vein thrombosis, cervical artery dissection, pituitary apoplexy, ischemic/hemorrhagic stroke, colloid cyst of the third ventricle, or intracranial infection [5, 74]. Meanwhile, TCH itself is attributable to sudden vasodilatation activating the perivascular sensory nerve fibers of the dorsal root of C2 as well as the trigeminal nerve [6]. In up to 15% of cases, RCVS may occur without the characteristic TCH [18, 72, 85]. Strongly suggestive of the presence of RCVS are recurrent TCH episodes (80%) in combination with initial brain parenchymal imaging showing no lesions or intracranial hemorrhage (up to 55%) [4]. Primary and secondary TCH onset may be spontaneous or associated with the following triggers: bathing, coughing, Valsalva maneuvers, and physical exertion [5, 19, 20, 55, 62, 63]. Based on TCH as the leading symptom, Fig. 2 represents the diagnostic workflow with regard to clinical management primarily for RCVS, with attention to the differential diagnoses.
This flowchart shows the diagnostic workflow from thunderclap headache (TCH) as a leading symptom to the detection of reversible cerebral vasoconstriction syndrome (RCVS) [2, 3, 5, 7, 9, 11, 98, 99, 105, 110, 112, 114, 116]. TCH thunderclap headache, CTA computed tomography angiography, MRA magnetic resonance angiography, TCD transcranial doppler, DSA digital subtraction angiography, ICB intracerebral hemorrhage, SAH subarachnoid hemorrhage, SVT sinus venous thrombosis, PACNS primary angiitis of the CNS, RCVS reversible cerebral vasoconstriction syndrome, CSF cerebrospinal fluid, i.v. intravenous, i.a. intra-arterial, p.o. per os
Focal neurological deficits (8–43% of cases) depend mainly on the complications of vasoconstriction [4, 40, 86]. Vasoconstriction even outlasts headache resolution and is therefore not a direct cause of headache [6, 40]. The focal deficits can also occur temporarily in the course of a transient ischemic attack (16%) [6]. Clinical symptoms depend primarily on the complications associated with RCVS, such as ischemic (39%) and hemorrhagic strokes (44%) and PRES (38%). The main presentations of PRES include encephalopathy (50–80%), seizures (60–75%), a dull, diffuse headache (50%), and visual disturbances (mainly Balint syndrome) (33%). These neurological symptoms are manifested acutely or subacutely, as in RCVS [76,77,78,79, 87]. However, the incidence of seizures in RCVS is lower, at 7–17% [4, 88]. Hemiparesis, hemianopsia, aphasia, dysarthria, and cortical blindness are often associated with intracranial hemorrhage and ischemic infarcts [4, 6, 23, 49, 57, 74].
Pathophysiology
The pathophysiology of RCVS is not fully understood at present. Two main hypotheses are currently being discussed: alterations in cerebral vascular tone and endothelial dysfunction [31, 32, 34,35,36]. Cerebral blood flow usually remains constant and thus ensures cerebral circulation [89]. This is achieved by changes in the cerebral arterioles [90]. The process of cerebral blood flow autoregulation can be disturbed by certain factors, such as sympathomimetic drugs or dysfunction in the autonomic nervous system, which have been shown to result in increased sympathetic activity as well as a reduced parasympathetic response by analyses of heart rate variability in RCVS patients [32]. Cerebral blood vessels are richly innervated by sympathetic nerve fibers [32, 90]. Sympathetic overactivity in RCVS is evident from its major risk factors of vasoactive agents (such as sympathomimetic and serotonergic drugs) and hormone-secreting tumors such as pheochromocytoma, as well as the 33–47% prevalence of systolic blood pressure surges in patients presenting with RCVS [1, 6, 49, 68]. This hyperperfusion overwhelms the body’s capacity for vascular autoregulation [35]. It results in endothelial dysfunction and breakdown of the blood‒brain barrier, causing not only increased vascular permeability but also vasoconstriction in the middle and distal arterial branches (PRES) or delayed proximal vasoconstriction after 1–2 weeks in RCVS [12, 33]. The endothelium usually regulates cerebral vascular tone by secreting certain vasoconstrictors and vasodilators [90]. The close association of RCVS with PRES, which can occur as a complication in up to 38% of RCVS cases, suggests a common pathophysiology [4, 33, 91]. There are also case reports of both syndromes affecting the same patient with the same risk factors (e.g., pre-eclampsia) [12, 15, 74]. A factor that appears to play a critical role in the development of both PRES and RCVS is the breakdown of the blood‒brain barrier, which can be visualized by contrast-enhanced FLAIR (CE-FLAIR) MRI [34]. Sixty-nine percent of patients with definite RCVS and 25% of patients with probable RCVS showed breakdown of the blood‒brain barrier on CE-FLAIR-MRI, even without the appearance of PRES [34]. In addition, blood–brain barrier dysfunction may be intrinsic to the pathophysiology of RCVS development [34].
Oxidative stress, hormonal and biochemical factors, and genetic predisposition also play critical roles [31, 92]. Cerebral vessel walls contain gonadal hormone receptors [93, 94]. Estrogen modulates vascular tone and cerebral blood flow via both nongenomic and genomic pathways in cerebral arteries and arterioles. This is achieved through, among other things, reduction of sympathetic tone; stimulation of endothelial nitrite oxide synthase (eNOS) gene expression; eNOS phosphorylation; and a shift in the prostanoid balance in favor of prostacyclin (PGI2), which has vasodilatory effects [95]. Additionally, sex steroids modulate the permeability of the blood‒brain barrier (BBB), on which estrogens have a marked effect [42, 96]. This could explain why the postpartum period, with its accompanying drop in estrogen levels, is an important trigger for RCVS (and PRES) [42]. However, according to a study by Topcuoglu and Singhal, different female subgroups (e.g., postpartum, nonpregnant, premenopausal, and postmenopausal) did not differ in terms of clinical and radiological outcomes or severity of RCVS [42]. Nevertheless, childbirth remains an important risk factor for the development of RCVS [42, 74]. A crucial factor in the development of pre-eclampsia is the role of the placenta, which secretes inflammatory cytokines as well as the proangiogenic proteins placental growth factor, its soluble receptor, and soluble endoglin into the maternal circulation [35, 97]. Thus, a systemic immune response with endothelial dysfunction and vasoconstriction follows. This may result in both PRES and RCVS [35].
Moreover, genetic predisposition is a possibility for cases in which no trigger can be found. According to certain studies, a gene polymorphism (Val66Met) in the brain-derived neurotrophic factor (BDNF) gene increases the severity of vasoconstriction in RCVS [92].
Complications and neuroimaging findings
Overall, 30–55% of RCVS patients show no abnormalities on initial brain MRI, and 22% show no changes on MR angiography [4, 6, 40, 98]. This reflects the dynamics of vasoconstriction. In one-fourth of patients who have normal brain MRI on presentation, no MRI abnormalities emerge during the further course of the disease [98]. In the days to weeks following the initial TCH event, up to 81% of RCVS patients develop brain lesions: infarcts (39%), intracranial hemorrhage (ICH; 44%, including cSAH in 34% of cases and lobar hemorrhage in 20%), PRES (38%), or a combination thereof (Fig. 3) [4, 6, 86, 88]. Usually, risk factors do not predict lesion type, with the exception of pre-eclampsia, which is often associated with RCVS and PRES, two conditions that seem to share a common pathophysiology involving blood‒brain barrier breakdown and endothelial dysfunction [33, 91, 99]. This leads to passive extravasation of fluid and proteins, causing vasogenic edema [12, 33, 91]. PRES represents a clinical and radiological syndrome; in nearly 65% of cases, it features bilateral parieto-occipital accentuation (Figs. 3a, 4), involves the cortex and underlying white matter and is often reversible in a few days [100]. In addition, other structures, such as the basal ganglia, frontal and temporal cortex, and brainstem, may be affected by vasogenic edema [30]. PRES appears on FLAIR and T2-weighted imaging (Figs. 3a, 4) and in the form of hyperintensities on apparent diffusion coefficient (ADC) mapping [85]. PRES-like vasogenic edema is detected in 9–38% of RCVS patients, but approximately 85% of PRES patients also develop multifocal vasoconstriction of the small peripheral arterioles on conventional angiography due to impaired cerebral autoregulation and endothelial dysfunction with a primary perfusion deficit [4, 33, 101]. Thus, PRES may additionally lead to cytotoxic edema due to ischemic stroke or may even lead to hemorrhage [12]. It is even postulated that RCVS and PRES represent two syndromes of a common disease with impaired cerebral vascular tone and endothelial dysfunction, as they share strikingly common pathophysiology and clinical symptoms [33].
The following magnetic resonance imaging (MRI) findings were obtained in a 55-year-old female patient with reversible cerebral vasoconstriction syndrome (RCVS). They reflect the most common complications of RCVS. a Occipital, predominantly right-lateralized fluid-attenuated inversion recovery (FLAIR) hyperintensities (transverse; yellow arrows), consistent with posterior reversible encephalopathy syndrome (PRES). b Hyperintensities on diffusion-weighted imaging (DWI) and FLAIR (hypointense on ADC (apparent diffusion coefficient) mapping) in the bilateral anterior circulation area (middle cerebral artery), resembling toxic edema (DWI, transverse; yellow arrows). c An atypically located intracerebral hemorrhage in the right frontal lobe on T2*-weighted MRI (transverse), also hyperintense on DWI (yellow ellipse in b, c)
This figure represents the characteristic MRI findings of posterior eversible encephalopathy syndrome (PRES), one of the most common complications of reversible cerebral vasoconstriction syndrome (RCVS). a–c Bi-occipital hyperintensities in different T2-weighted images without ADC (apparent diffusion coefficient) hypointensities, corresponding to PRES (yellow arrows). a Proton-density-weighted (PDw) MRI in the transverse plane, b DWI in the transverse plane, and c FLAIR in the coronal plane
There is a marked temporal discrepancy between the occurrence of ischemic and hemorrhagic events [6, 40]. The severity of vasoconstriction peaks 9–13 days after the initial TCH event [6, 72]. Seventy-four percent of RCVS patients were found to have unusual severe headache approximately 9 days before ischemic stroke [72]. The process of vasoconstriction, caused by defective autoregulation of the vessel wall, begins in the distal capillary bed and migrates proximally [99]. This also reflects the dynamics of angiographic findings [102]. Thus, after approximately 2 weeks, the first and second segments of the great arteries become affected [99]. Maximal vasoconstriction (≥ 75%) in the P2 or M1 segment on MR-TOF angiography correlate significantly with PRES and ischemic stroke, respectively [86]. A study detected a mean of 4.52 stenoses per patient, which were fully reversible in 3–6 months [72]. In the clinical–radiological condition of RCVS, two infarct types are seen: smaller, peripheral, cortical infarcts, which occur rather early in the time course and are due to a primary perfusion deficit caused by maladaptive autoregulation in the arteriolar area, and larger, wedge-shaped watershed infarcts that are due to severe hypoperfusion distal to severe vasoconstriction (Fig. 3b) [11, 40, 98]. Ischemic strokes occur singly rather than in groups despite widespread vasoconstriction [98]. This further reflects the dynamics of the disease and prevents more pronounced infarction [98].
In general, the rate of hemorrhage as a complication is higher in RCVS than in PRES. Intracerebral hemorrhages occur in up to 44% of RCVS cases, with the most common form (22–35%) being convexity subarachnoid hemorrhage (cSAH) [4, 6, 40, 88]. Consequently, the most common complication of RCVS leading to hospitalization is ICH (Fig. 3c) [88]. Female gender and a history of migraine are considered independent risk factors for the occurrence of any type of hemorrhage in RCVS [40, 88]. Vascular aberrations start days before the attack and are characterized by dynamics of vasoconstriction and vasodilation [40, 99]. Abrupt stretching of the small pial cortical vessels may lead to reperfusion injury, which results in rupture of the small vessels and thus secondary hemorrhage [40, 88]. Hemorrhagic manifestations usually begin to appear 2–4 days after the initial TCH attack [40]. SAH in RCVS is usually confined to 1–3 sulci, overlying them, and is therefore overlooked in many cases [103, 104]. The most commonly affected site is the subarachnoid space over the frontal lobe (51%), followed by the anterior parietal region (21%) [104]. FLAIR-MRI characteristically shows what is known as the dot sign, which consists of punctate hyperintensities [98]. These hyperintensities are located in the area of the sulci of both hemispheres in RCVS patients and reflect the vasodilatation of the small cortical vessels [4, 98]. This finding supports the hypothesis that hemorrhages in RCVS are driven by cerebral autoregulation failure and sudden hypertension with resulting vasoconstriction and vasodilation, starting distally [90, 98].
Differential diagnoses and neuroimaging findings
TCH is the cardinal symptom of RCVS and the main symptom of a ruptured brain aneurysm causing SAH [103]. As mentioned under the heading "Complications", RCVS itself can lead to subarachnoid hemorrhage [40]. However, in up to 85% of patients, SAH is a consequence of aneurysm rupture [6, 105]. A less common cause of SAH may be RCVS itself. In addition, it must be mentioned that unruptured aneurysms may occur in 3–8% by chance [103, 106]. An important and difficult point emerges, namely, the distinction between SAH secondary to RCVS and SAH due to a ruptured aneurysm (aSAH) [107]. This distinction is of enormous relevance, because RCVS is actually a benign entity, in contrast to aneurysm, a more life-threatening cause of SAH. A major distinguishing feature of both entities is that the blood component in the subarachnoid space due to RCVS is unilaterally localized, covering only 1–3 adjacent sulci in up to 83% of patients in a retrospective study by Kumar et al. [104]. When SAH is localized in this way, it is sometimes referred to as cortical or convexity SAH (cSAH) [6, 105, 107, 108]. In the context of aSAH, blood is distributed across a wider range of locations, including the sylvian fissure and basal cisterns, and ventricular collapse can also be observed [103]. Vasospasm due to aSAH usually occurs unilaterally around the SAH and involves 1–2 medium-sized arteries [107]. Long, smooth regions of vasoconstriction, primarily of the proximal cisternal segments, usually peak from day 4 to day 14 after aneurysm rupture, in contrast to vasospasm in RCVS, which is widely distributed before cSAH occurs [107]. In addition, it seems unlikely that the amount of subarachnoid blood in aSAH can cause vasospasm scattered throughout the brain vessels. This is in contrast to RCVS, in which there is often minimal subarachnoid blood content, but vasospasm is widespread and multifocal with involvement of bilateral arteries [109]. Furthermore, approximately 80% of aSAH patients develop only one episode of TCH, whereas 85–100% of RCVS patients develop multiple episodes (up to 4) in the first few days [103].
On the basis of vascular stenoses, one can identify another differential diagnosis, namely, primary angiitis of the central nervous system (PACNS) [110]. In contrast to the severe, smooth, tapering, widespread vasoconstrictions, and vasodilatations of the vessels of the circle of Willis primarily after passage through the dura in RCVS (Fig. 5a), the irregular, notched, eccentric stenoses and ectasias in PACNS result from chronic vessel wall inflammation and destruction with distal cut-offs [11, 98, 111, 112]. Vasoconstriction in RCVS is reversible, which is also reflected in the established diagnostic criteria (Table 2) [11]. Whereas digital subtraction angiography (DSA) often fails to show any abnormalities in PACNS, it is always abnormal in RCVS (Fig. 5c) [11, 98, 111]. The reversibility of stenoses is an important point of differentiation from PACNS, which is why calcium channel antagonists can also be given diagnostically in the context of conventional angiography (Fig. 5d) [9, 10, 113]. PACNS is accompanied by dull persistent headache in 51% of cases [98]; TCH occurs in only approximately 6% [98]. The analysis of cerebrospinal fluid (CSF) is an indispensable first diagnostic tool for headache diagnosis [114]. Patients with PACNS present with pathological CSF findings in up to 90% of cases [4, 111]. Important alterations include moderate pleocytosis with an increased protein concentration, occasional intrathecal IgG synthesis, and the appearance of oligoclonal bands [111]. However, abnormal CSF findings are atypical in RCVS and, if present, include minimal pleocytosis and slightly elevated protein levels [110, 114]. In a retrospective study by Singhal et al. comparing PACNS and RCVS patients in different aspects, such as symptoms and brain and angiographic findings, one-quarter of all RCVS patients showed no abnormalities regarding the brain parenchyma on initial MRI despite widespread and severe vessel narrowing [98]. Moreover, RCVS patients were significantly younger, had a 2.6-fold higher proportion of women, and had a higher rate of recent intake of vasoconstrictive or illicit drugs [98]. Finally, RCVS patients are more likely to develop wedge-shaped infarcts in watershed regions, in contrast to PACNS patients, nearly 76% of whom have small and widespread acute, subacute, or chronic infarcts with diffuse T2-hyperintense white matter lesions [98, 111, 112]. Only approximately 2–9% of PACNS patients develop parenchymal hemorrhage or cSAH, whereas PRES has not yet been reported in PACNS [98]. The brainstem and deep gray matter are involved in up to 74% of infarcts in PACNS, which rarely have a predilection for ischemia in RCVS [98]. The most important distinguishing diagnostic features are summarized in Table 5.
Typical angiographic findings in the patient with reversible cerebral vasoconstriction syndrome (RCVS) shown in Fig. 3. a MR-TOF angiography with diffuse regions of vasoconstriction (creating a “sausage-on-a-string” appearance) in all territories (yellow arrows). b Full remission of vasoconstrictions on MR-TOF angiography 3 weeks after an oral nimodipine regimen was started. c Digital subtraction angiography (DSA) via left vertebral artery in the same patient. Multiple vasospasms in the area of the left posterior cerebral artery and left superior cerebellar artery (yellow arrows) as well as in the left anterior cerebral artery (not shown) can be detected. d Diagnostic DSA after intra-arterial administration of 1 mg nimodipine. Complete reversibility of the vasospasms was observed
The modified Rankin scale (mRS) score at discharge was between 0 and 1 in 90% of RCVS patients, which was significantly higher than the proportion in PACNS patients (70%) [98]. To date, there are no official guidelines for RCVS therapy. Many case reports and small prospective studies showed that the use of calcium channel antagonists (nimodipine, nifedipine, or verapamil) as early as possible could have favorable effects on the clinical course (Fig. 5b) [1, 4, 8, 9, 18, 115, 116]. In a prospective study by Cho et al., 86.6% of clinically and radiologically confirmed RCVS patients achieved remission of TCH [7].
Patients are often subjected to risky diagnostics, especially biopsies of the vessel wall, or are started on immunosuppressive therapy, primarily with glucocorticoids, out of concern that PACNS may be overlooked [98, 110]. In a study, PACNS and RCVS patients were compared in terms of clinical, laboratory, and radiological aspects [98]. Criteria for rapid bedside diagnosis were developed. The authors reported that these criteria achieved a specificity and a positive predictive value of 100% for RCVS in patients with recurrent TCH episodes or a single TCH episode combined with either border zone infarcts, evidence of PRES, or even normal intracranial imaging [98]. In contrast, inflammatory markers in CSF plus infarcts in the brainstem or deep gray matter achieve a positive predictive value of 100% for PACNS [98]. A detailed differential diagnosis is also important with regard to the upcoming therapeutic options. It has already been demonstrated that treatment with glucocorticoids in RCVS patients is an independent predictor of clinical–radiological deterioration with a poor outcome [4, 37, 117]. Clinical worsening was associated with new infarcts in 44–70% of patients. Independently, the occurrence of ischemic infarcts in baseline imaging is a predictor of poor outcome [38]. Forty-seven percent of glucocorticoid-treated RCVS patients had an mRS of 4–6 at discharge, compared to 17% in the untreated group [37]. One case report even discussed low-dose steroid treatment in the context of rheumatoid arthritis as a precipitating factor for RCVS [18].
Prognosis and conclusion
Despite the occurrence of serious complications, the prognosis of RCVS in both adults and children is very good. Over 90% of patients have a good outcome, defined as 0–1 on the mRS [4, 43, 98]. A worse outcome is associated with severe infarcts early in the disease history and pronounced focal neurological deficits at baseline, leading to death [38]. As mentioned above, glucocorticoids worsen the outcome; in contrast, vasoactive agents such as SSRIs and triptans are associated with worsening vasospasm or new neurological deficits, underlining the role of serotonergic drugs in the pathophysiology, while these agents do not worsen the clinical outcome [4, 37]. Overall, 97.5% of patients in a retrospective study were functionally independent according to the Barthel index [118]. Even after TCH was no longer present and vasoconstriction was reversed, 53% of patients continued to have mild-to-moderate headache. Of these, the majority were triggered by migraine and vasoactive medication [118].
However, the pathophysiology is still not fully understood. Further basic studies are necessary for a better understanding and possibly for further therapeutic options, as some fulminant cases, e.g., in the postpartum state, can still lead to death or severe disabilities [47].
Data and material availability
The material used during the current work is available from the corresponding author on reasonable request.
Abbreviations
- TCH:
-
Thunderclap headache
- RCVS:
-
Reversible cerebral vasoconstriction syndrome
- PRES:
-
Posterior reversible encephalopathy syndrome
- CT:
-
Computer tomography
- MRI:
-
Magnetic resonance tomography
- PACNS:
-
Primary angiitis of the central nervous system
- SAH:
-
Subarachnoid hemorrhage
- cSAH:
-
Convexity subarachnoid hemorrhage
- aSAH:
-
Subarachnoid hemorrhage due to aneurysm rupture
- ICH:
-
Intracranial hemorrhage
- eNOS:
-
Endothelial nitrite oxide synthase
- PGI2:
-
Prostacyclin
- BBB:
-
Blood–brain barrier
- SSRI:
-
Selective serotonin reuptake inhibitors
- SNRI:
-
Serotonin and noradrenaline reuptake inhibitors
- PPA:
-
Postpartum angiopathy
- ACE2:
-
Angiotensin-converting-enzyme 2
- (CE-) FLAIR:
-
(Contrast-enhanced) fluid-attenuated inversion recovery
- BDNF:
-
Brain-derived neurotrophic factor
- ICDH-3:
-
International Classification of Headache Disorders 3 (beta version) criteria
- CSF:
-
Cerebrospinal fluid
- OCB:
-
Oligoclonal bands
- mRS:
-
Modified Rankin scale
References
Choi HA, Lee MJ, Choi H, Chung CS (2018) Characteristics and demographics of reversible cerebral vasoconstriction syndrome: a large prospective series of Korean patients. Cephalalgia 38:765–775. https://doi.org/10.1177/0333102417715223
Wolff V, Ducros A (2016) Reversible cerebral vasoconstriction syndrome without typical thunderclap headache. Headache 56:674–687. https://doi.org/10.1111/head.12794
Ducros A (2012) Reversible cerebral vasoconstriction syndrome. Lancet Neurol 11:906–917. https://doi.org/10.1016/S1474-4422(12)70135-7
Singhal AB, Hajj-Ali RA, Topcuoglu MA, Fok J, Bena J, Yang D, Calabrese LH (2011) Reversible cerebral vasoconstriction syndromes: analysis of 139 cases. Arch Neurol 68:1005–1012. https://doi.org/10.1001/archneurol.2011.68
Schwedt TJ, Matharu MS, Dodick DW (2006) Thunderclap headache. Lancet Neurol 5:621–631. https://doi.org/10.1016/s1474-4422(06)70497-5
Ducros A, Boukobza M, Porcher R, Sarov M, Valade D, Bousser MG (2007) The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients. Brain 130:3091–3101. https://doi.org/10.1093/brain/awm256
Cho S, Lee MJ, Chung CS (2019) Effect of nimodipine treatment on the clinical course of reversible cerebral vasoconstriction syndrome. Front Neurol 10:644. https://doi.org/10.3389/fneur.2019.00644
Chen SP, Fuh JL, Lirng JF, Chang FC, Wang SJ (2006) Recurrent primary thunderclap headache and benign CNS angiopathy: spectra of the same disorder? Neurology 67:2164–2169. https://doi.org/10.1212/01.wnl.0000249115.63436.6d
Linn J, Fesl G, Ottomeyer C, Straube A, Dichgans M, Bruckmann H, Pfefferkorn T (2011) Intra-arterial application of nimodipine in reversible cerebral vasoconstriction syndrome: a diagnostic tool in select cases? Cephalalgia 31:1074–1081. https://doi.org/10.1177/0333102410394673
Strunk D, Veltkamp R, Meuth SG, Chapot R, Kraemer M (2022) Intra-arterial application of nimodipine in reversible cerebral vasoconstriction syndrome: a neuroradiological method to help differentiate from primary central nervous system vasculitis. Neurol Res Pract 4:8. https://doi.org/10.1186/s42466-022-00173-0
Calabrese LH, Dodick DW, Schwedt TJ, Singhal AB (2007) Narrative review: reversible cerebral vasoconstriction syndromes. Ann Intern Med 146:34–44. https://doi.org/10.7326/0003-4819-146-1-200701020-00007
Singhal AB (2004) Postpartum angiopathy with reversible posterior leukoencephalopathy. Arch Neurol 61:411–416. https://doi.org/10.1001/archneur.61.3.411
Singhal AB, Caviness VS, Begleiter AF, Mark EJ, Rordorf G, Koroshetz WJ (2002) Cerebral vasoconstriction and stroke after use of serotonergic drugs. Neurology 58:130–133. https://doi.org/10.1212/wnl.58.1.130
Meschia JF, Malkoff MD, Biller J (1998) Reversible segmental cerebral arterial vasospasm and cerebral infarction: possible association with excessive use of sumatriptan and Midrin. Arch Neurol 55:712–714. https://doi.org/10.1001/archneur.55.5.712
Fletcher JJ, Kramer AH, Bleck TP, Solenski NJ (2009) Overlapping features of eclampsia and postpartum angiopathy. Neurocrit Care 11:199–209. https://doi.org/10.1007/s12028-009-9221-0
Kass-Hout T, Kass-Hout O, Sun CH, Kass-Hout T, Ramakrishnan P, Nahab F, Nogueira R, Gupta R (2015) A novel approach to diagnose reversible cerebral vasoconstriction syndrome: a case series. J Stroke Cerebrovasc Dis 24:e31-37. https://doi.org/10.1016/j.jstrokecerebrovasdis.2014.08.023
Singhal AB, Bernstein RA (2005) Postpartum angiopathy and other cerebral vasoconstriction syndromes. Neurocrit Care 3:91–97. https://doi.org/10.1385/ncc:3:1:091
Erhart DK, Ludolph AC, Althaus K (2022) Cerebral infarctions following an increase in corticosteroids: an atypical case of reversible cerebral vasoconstriction syndrome. J Neurol. https://doi.org/10.1007/s00415-022-11170-3
Komatsu T, Kimura T, Yagishita A, Takahashi K, Koide R (2014) A case of reversible cerebral vasoconstriction syndrome presenting with recurrent neurological deficits: Evaluation using noninvasive arterial spin labeling MRI. Clin Neurol Neurosurg 26:96–98. https://doi.org/10.1016/j.clineuro.2014.08.023
Theeler BJ, Krasnokutsky MV, Scott BR (2010) Exertional reversible cerebral vasoconstriction responsive to verapamil. Neurol Sci 31:773–775. https://doi.org/10.1007/s10072-010-0226-4
Uhegwu N, Bashir A, Hussain M, Dababneh H, Misthal S, Cohen-Gadol A (2015) Marijuana induced reversible cerebral vasoconstriction syndrome. J Vasc Interv Neurol 8:36–38
Roberts A, Sowers N (2020) A case of reversible cerebral vasoconstriction syndrome in a healthy adult male. Cureus 12:e8374. https://doi.org/10.7759/cureus.8374
Marder CP, Donohue MM, Weinstein JR, Fink KR (2012) Multimodal imaging of reversible cerebral vasoconstriction syndrome: a series of 6 cases. Am J Neuroradiol 33:1403. https://doi.org/10.3174/ajnr.A2964
Baharith H, Zarrin A (2016) Khat—a new precipitating factor for reversible cerebral vasoconstriction syndrome: a case report. J Med Case Rep 10:351. https://doi.org/10.1186/s13256-016-1155-5
Calic Z, Choong H, Schlaphoff G, Cappelen-Smith C (2014) Reversible cerebral vasoconstriction syndrome following indomethacin. Cephalalgia 34:1181–1186. https://doi.org/10.1177/0333102414530526
Palma JA, Fontes-Villalba A, Irimia P, Garcia-Eulate R, Martinez-Vila E (2012) Reversible cerebral vasoconstriction syndrome induced by adrenaline. Cephalalgia 32:500–504. https://doi.org/10.1177/0333102412444011
Lopez-Valdes E, Chang HM, Pessin MS, Caplan LR (1997) Cerebral vasoconstriction after carotid surgery. Neurology 49:303–304. https://doi.org/10.1212/wnl.49.1.303
Rosenbloom MH, Singhal AB (2007) CT angiography and diffusion-perfusion MR imaging in a patient with ipsilateral reversible cerebral vasoconstriction after carotid endarterectomy. AJNR Am J Neuroradiol 28:920–922
Chen SP, Fuh JL, Wang SJ (2011) Reversible cerebral vasoconstriction syndrome: current and future perspectives. Expert Rev Neurother 11:1265–1276. https://doi.org/10.1586/ern.11.112
Sadek AR, Waters RJ, Sparrow OC (2012) Posterior reversible encephalopathy syndrome: a case following reversible cerebral vasoconstriction syndrome masquerading as subarachnoid haemorrhage. Acta Neurochir (Wien) 154:413–416. https://doi.org/10.1007/s00701-011-1268-y
Chen SP, Wang YF, Huang PH, Chi CW, Fuh JL, Wang SJ (2014) Reduced circulating endothelial progenitor cells in reversible cerebral vasoconstriction syndrome. J Headache Pain 15:82. https://doi.org/10.1186/1129-2377-15-82
Chen SP, Yang AC, Fuh JL, Wang SJ (2013) Autonomic dysfunction in reversible cerebral vasoconstriction syndromes. J Headache Pain 14:94. https://doi.org/10.1186/1129-2377-14-94
Feil K, Forbrig R, Thaler FS, Conrad J, Heck S, Dorn F, Pfister HW, Straube A (2017) Reversible cerebral vasoconstriction syndrome and posterior reversible encephalopathy syndrome associated with intracranial hypotension. Neurocrit Care 26:103–108. https://doi.org/10.1007/s12028-016-0320-4
Lee MJ, Cha J, Choi HA, Woo SY, Kim S, Wang SJ, Chung CS (2017) Blood-brain barrier breakdown in reversible cerebral vasoconstriction syndrome: implications for pathophysiology and diagnosis. Ann Neurol 81:454–466. https://doi.org/10.1002/ana.24891
Pop A, Carbonnel M, Wang A, Josserand J, Auliac SC, Ayoubi JM (2019) Posterior reversible encephalopathy syndrome associated with reversible cerebral vasoconstriction syndrome in a patient presenting with postpartum eclampsia: a case report. J Gynecol Obstet Hum Reprod 48:431–434. https://doi.org/10.1016/j.jogoh.2019.03.019
Chen SP, Fuh JL, Lirng JF, Wang SJ (2012) Hyperintense vessels on flair imaging in reversible cerebral vasoconstriction syndrome. Cephalalgia 32:271–278. https://doi.org/10.1177/0333102412437387
Singhal AB, Topcuoglu MA (2017) Glucocorticoid-associated worsening in reversible cerebral vasoconstriction syndrome. Neurology 88:228–236. https://doi.org/10.1212/wnl.0000000000003510
Robert T, Kawkabani Marchini A, Oumarou G, Uské A (2013) Reversible cerebral vasoconstriction syndrome identification of prognostic factors. Clin Neurol Neurosurg 115:2351–2357. https://doi.org/10.1016/j.clineuro.2013.08.014
Kirton A, Diggle J, Hu W, Wirrell E (2006) A pediatric case of reversible segmental cerebral vasoconstriction. Can J Neurol Sci 33:250–253. https://doi.org/10.1017/s0317167100005096
Ducros A, Fiedler U, Porcher R, Boukobza M, Stapf C, Bousser MG (2010) Hemorrhagic manifestations of reversible cerebral vasoconstriction syndrome: frequency, features, and risk factors. Stroke 41:2505–2511. https://doi.org/10.1161/strokeaha.109.572313
Garg A, Rocha M, Starr M, Ortega-Gutierrez S (2021) Predictors and outcomes of hemorrhagic stroke in reversible cerebral vasoconstriction syndrome. J Neurol Sci 421:117312. https://doi.org/10.1016/j.jns.2021.117312
Topcuoglu MA, McKee KE, Singhal AB (2016) Gender and hormonal influences in reversible cerebral vasoconstriction syndrome. Eur Stroke J 1:199–204. https://doi.org/10.1177/2396987316656981
Coffino SW, Fryer RH (2017) Reversible cerebral vasoconstriction syndrome in pediatrics: a case series and review. J Child Neurol 32:614–623. https://doi.org/10.1177/0883073817696817
Guerriero RM, Rivkin MJ (2015) Reversible vasoconstriction syndrome involving the basilar artery in an adolescent: imaging and clinical features. Pediatr Neurol 52:635–637. https://doi.org/10.1016/j.pediatrneurol.2015.02.015
Yoshioka S, Takano T, Ryujin F, Takeuchi Y (2012) A pediatric case of reversible cerebral vasoconstriction syndrome with cortical subarachnoid hemorrhage. Brain Dev 34:796–798. https://doi.org/10.1016/j.braindev.2012.01.001
Jensen J, Leonard J, Salottolo K, McCarthy K, Wagner J, Bar-Or D (2018) The epidemiology of reversible cerebral vasoconstriction syndrome in patients at a colorado comprehensive stroke center. J Vasc Interv Neurol 10:32–38
Fugate JE, Wijdicks EF, Parisi JE, Kallmes DF, Cloft HJ, Flemming KD, Giraldo EA, Rabinstein AA (2012) Fulminant postpartum cerebral vasoconstriction syndrome. Arch Neurol 69:111–117. https://doi.org/10.1001/archneurol.2011.811
Hadhiah KM, Alshagawi ZA, Alzahrani SK, Alrayes MM, Aldandan HW (2021) Reversible cerebral vasoconstriction syndrome in a background of eclampsia responding to milrinone infusion. Am J Case Rep 22:e934528. https://doi.org/10.12659/ajcr.934528
John S, Donnelly M, Uchino K (2013) Catastrophic reversible cerebral vasoconstriction syndrome associated with serotonin syndrome. Headache 53:1482–1487. https://doi.org/10.1111/head.12202
Kato Y, Hayashi T, Mizuno S, Horiuchi Y, Ohira M, Tanahashi N, Takao M (2016) Triptan-induced reversible cerebral vasoconstriction syndrome: two case reports with a literature review. Intern Med 55:3525–3528. https://doi.org/10.2169/internalmedicine.55.7185
Manning T, Bartow C, Dunlap M, Kiehl R, Kneale H, Walker A (2021) Reversible cerebral vasoconstriction syndrome associated with fluoxetine. J Acad Consult Liaison Psychiatry 62:634–644. https://doi.org/10.1016/j.jaclp.2021.07.013
Ray S, Kamath VV, Raju PA, Kn R (2022) Fulminant reversible cerebral vasoconstriction syndrome in breakthrough COVID 19 infection. J Stroke Cerebrovasc Dis 31:106238. https://doi.org/10.1016/j.jstrokecerebrovasdis.2021.106238
Short K, Emsley HCA (2021) Illicit drugs and reversible cerebral vasoconstriction syndrome. Neurohospitalist 11:40–44. https://doi.org/10.1177/1941874420953051
Kraemer M, Weber R, Herold M, Berlit P (2015) Reversible cerebral vasoconstriction syndrome associated with fingolimod treatment in relapsing-remitting multiple sclerosis three months after childbirth. Mult Scler 21:1473–1475. https://doi.org/10.1177/1352458515600249
Liao YC, Fuh JL, Lirng JF, Lu SR, Wu ZA, Wang SJ (2003) Bathing headache: a variant of idiopathic thunderclap headache. Cephalalgia 23:854–859. https://doi.org/10.1046/j.1468-2982.2003.00603.x
Al-Mufti F, Amuluru K, Changa A, Lander M, Patel N, Wajswol E, Al-Marsoummi S, Alzubaidi B, Singh IP, Nuoman R, Gandhi C (2017) Traumatic brain injury and intracranial hemorrhage-induced cerebral vasospasm: a systematic review. Neurosurg Focus 43:E14. https://doi.org/10.3171/2017.8.Focus17431
Doss-Esper CE, Singhal AB, Smith MS, Henderson GV (2005) Reversible posterior leukoencephalopathy, cerebral vasoconstriction, and strokes after intravenous immune globulin therapy in guillain-barre syndrome. J Neuroimaging 15:188–192. https://doi.org/10.1177/1051228404273820
Boughammoura A, Touzé E, Oppenheim C, Trystram D, Mas JL (2003) Reversible angiopathy and encephalopathy after blood transfusion. J Neurol 250:116–118. https://doi.org/10.1007/s00415-003-0940-4
Saito K, Shimizu Y, Higuma M, Kubodera T, Wada Y (2019) Posterior reversible encephalopathy syndrome and reversible cerebral vasoconstriction syndrome after rapid blood transfusion. Intern Med 58:2225–2230. https://doi.org/10.2169/internalmedicine.1768-18
Togha M, Babaei M, Ghelichi PG (2021) Reversible cerebral vasoconstriction syndrome (RCVS): an interesting case report. J Headache Pain 22:20. https://doi.org/10.1186/s10194-021-01225-7
Verillaud B, Ducros A, Massiou H, Huy PT, Bousser MG, Herman P (2010) Reversible cerebral vasoconstriction syndrome in two patients with a carotid glomus tumour. Cephalalgia 30:1271–1275. https://doi.org/10.1177/0333102410365107
Mak W, Tsang KL, Tsoi TH, Au Yeung KM, Chan KH, Cheng TS, Cheung TF, Ho SL (2005) Bath-related headache. Cephalalgia 25:191–198. https://doi.org/10.1111/j.1468-2982.2004.00832.x
Kato Y, Hayashi T, Sano H, Kato R, Tanahashi N, Takao M (2018) Cough headache presenting with reversible cerebral vasoconstriction syndrome. Intern Med 57:1459–1461. https://doi.org/10.2169/internalmedicine.0061-17
Wong SH, Dougan C, Chatterjee K, Fletcher NA, White RP (2009) Recurrent thunderclap headaches and multilobar intracerebral haemorrhages: two cases of reversible cerebral vasoconstriction syndrome (RCVS). Cephalalgia 29:791–795. https://doi.org/10.1111/j.1468-2982.2008.01805.x
Hu CM, Lin YJ, Fan YK, Chen SP, Lai TH (2010) Isolated thunderclap headache during sex: orgasmic headache or reversible cerebral vasoconstriction syndrome? J Clin Neurosci 17:1349–1351. https://doi.org/10.1016/j.jocn.2010.01.052
Headache Classification Committee of the International Headache Society (IHS) (2013) The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 33:629–808. https://doi.org/10.1177/0333102413485658
Katz BS, Fugate JE, Ameriso SF, Pujol-Lereis VA, Mandrekar J, Flemming KD, Kallmes DF, Rabinstein AA (2014) Clinical worsening in reversible cerebral vasoconstriction syndrome. JAMA Neurol 71:68–73. https://doi.org/10.1001/jamaneurol.2013.4639
English SW, Nasr DM (2019) Thunderclap headache and cerebral vasoconstriction secondary to pheochromocytoma. JAMA Neurol 76:502–503. https://doi.org/10.1001/jamaneurol.2019.0001
Zeitouni D, Parish JM, Smith M, Stetler WR, Bernard JD (2021) Reversible cerebral vasoconstriction syndrome successfully treated by intrathecal nicardipine. Clin Neurol Neurosurg 206:106705. https://doi.org/10.1016/j.clineuro.2021.106705
Cantu C, Arauz A, Murillo-Bonilla LM, López M, Barinagarrementeria F (2003) Stroke associated with sympathomimetics contained in over-the-counter cough and cold drugs. Stroke 34:1667–1672. https://doi.org/10.1161/01.Str.0000075293.45936.Fa
Wolff V, Lauer V, Rouyer O, Sellal F, Meyer N, Raul JS, Sabourdy C, Boujan F, Jahn C, Beaujeux R, Marescaux C (2011) Cannabis use, ischemic stroke, and multifocal intracranial vasoconstriction: a prospective study in 48 consecutive young patients. Stroke 42:1778–1780. https://doi.org/10.1161/strokeaha.110.610915
Wolff V, Armspach JP, Lauer V, Rouyer O, Ducros A, Marescaux C, Gény B (2015) Ischaemic strokes with reversible vasoconstriction and without thunderclap headache: a variant of the reversible cerebral vasoconstriction syndrome? Cerebrovasc Dis 39:31–38. https://doi.org/10.1159/000369776
Koopman K, Teune LK, Mt L, Uyttenboogaart M, Vroomen PC, Keyser JD, Luijckx GJ (2008) An often unrecognized cause of thunderclap headache: reversible cerebral vasoconstriction syndrome. J Headache Pain 9:389–391. https://doi.org/10.1007/s10194-008-0068-0
Singhal AB, Kimberly WT, Schaefer PW, Hedley-Whyte ET (2009) Case records of the Massachusetts General Hospital. case 8–2009. A 36-year-old woman with headache, hypertension, and seizure 2 weeks post partum. N Engl J Med 360:1126–1137. https://doi.org/10.1056/NEJMcpc0809063
Lee KY, Sohn YH, Kim SH, Sunwoo IN (2000) Basilar artery vasospasm in postpartum cerebral angiopathy. Neurology 54:2003–2005. https://doi.org/10.1212/wnl.54.10.2003
Burnett MM, Hess CP, Roberts JP, Bass NM, Douglas VC, Josephson SA (2010) Presentation of reversible posterior leukoencephalopathy syndrome in patients on calcineurin inhibitors. Clin Neurol Neurosurg 112:886–891. https://doi.org/10.1016/j.clineuro.2010.07.023
Fugate JE, Claassen DO, Cloft HJ, Kallmes DF, Kozak OS, Rabinstein AA (2010) Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc 85:427–432. https://doi.org/10.4065/mcp.2009.0590
Li R, Mitchell P, Dowling R, Yan B (2013) Is hypertension predictive of clinical recurrence in posterior reversible encephalopathy syndrome? J Clin Neurosci 20:248–252. https://doi.org/10.1016/j.jocn.2012.02.023
Liman TG, Bohner G, Heuschmann PU, Endres M, Siebert E (2012) The clinical and radiological spectrum of posterior reversible encephalopathy syndrome: the retrospective Berlin PRES study. J Neurol 259:155–164. https://doi.org/10.1007/s00415-011-6152-4
Arandela K, Samudrala S, Abdalkader M, Anand P, Daneshmand A, Dasenbrock H, Nguyen T, Ong C, Takahashi C, Shulman J, Babi MA, Sivakumar S, Shah N, Jain S, Anand S, Nobleza COS, Shekhar S, Venkatasubramanian C, Salahuddin H, Taqi MA, Nour HA, Nofar JB, Cervantes-Arslanian AM (2021) Reversible cerebral vasoconstriction syndrome in patients with coronavirus disease: a multicenter case series. J Stroke Cerebrovasc Dis 30:106118. https://doi.org/10.1016/j.jstrokecerebrovasdis.2021.106118
Mansoor T, Alsarah AA, Mousavi H, Khader Eliyas J, Girotra T, Hussein O (2021) COVID-19 associated reversible cerebral vasoconstriction syndrome successfully treated with nimodipine and aspirin. J Stroke Cerebrovasc Dis 30:105822. https://doi.org/10.1016/j.jstrokecerebrovasdis.2021.105822
Dakay K, Kaur G, Gulko E, Santarelli J, Bowers C, Mayer SA, Gandhi CD, Al-Mufti F (2020) Reversible cerebral vasoconstriction syndrome and dissection in the setting of COVID-19 infection. J Stroke Cerebrovasc Dis 29:105011. https://doi.org/10.1016/j.jstrokecerebrovasdis.2020.105011
Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS (2020) Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med 46:586–590. https://doi.org/10.1007/s00134-020-05985-9
Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, Kneen R, Defres S, Sejvar J, Solomon T (2020) Neurological associations of COVID-19. Lancet Neurol 19:767–783. https://doi.org/10.1016/s1474-4422(20)30221-0
Wagih A, Mohsen L, Rayan MM, Hasan MM, Al-Sherif AH (2015) Posterior reversible encephalopathy syndrome (PRES): restricted diffusion does not necessarily mean irreversibility. Pol J Radiol 80:210–216. https://doi.org/10.12659/pjr.893460
Chen SP, Fuh JL, Wang SJ, Chang FC, Lirng JF, Fang YC, Shia BC, Wu JC (2010) Magnetic resonance angiography in reversible cerebral vasoconstriction syndromes. Ann Neurol 67:648–656. https://doi.org/10.1002/ana.21951
Legriel S, Schraub O, Azoulay E, Hantson P, Magalhaes E, Coquet I, Bretonniere C, Gilhodes O, Anguel N, Megarbane B, Benayoun L, Schnell D, Plantefeve G, Charpentier J, Argaud L, Mourvillier B, Galbois A, Chalumeau-Lemoine L, Rivoal M, Durand F, Geffroy A, Simon M, Stoclin A, Pallot JL, Arbelot C, Nyunga M, Lesieur O, Troché G, Bruneel F, Cordoliani YS, Bedos JP, Pico F (2012) Determinants of recovery from severe posterior reversible encephalopathy syndrome. PLoS ONE 7:e44534. https://doi.org/10.1371/journal.pone.0044534
Patel SD, Topiwala K, Saini V, Patel N, Pervez M, Al-Mufti F, Hassan AE, Khandelwal P, Starke RM (2021) Hemorrhagic reversible cerebral vasoconstriction syndrome: a retrospective observational study. J Neurol 268:632–639. https://doi.org/10.1007/s00415-020-10193-y
Hamner JW, Tan CO (2014) Relative contributions of sympathetic, cholinergic, and myogenic mechanisms to cerebral autoregulation. Stroke 45:1771–1777. https://doi.org/10.1161/strokeaha.114.005293
Bartynski WS (2008) Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol 29:1043–1049. https://doi.org/10.3174/ajnr.A0929
Marra A, Vargas M, Striano P, Del Guercio L, Buonanno P, Servillo G (2014) Posterior reversible encephalopathy syndrome: the endothelial hypotheses. Med Hypothes 82:619–622. https://doi.org/10.1016/j.mehy.2014.02.022
Chen SP, Fuh JL, Wang SJ, Tsai SJ, Hong CJ, Yang AC (2011) Brain-derived neurotrophic factor gene Val66Met polymorphism modulates reversible cerebral vasoconstriction syndromes. PLoS ONE 6:e18024. https://doi.org/10.1371/journal.pone.0018024
Ohtsuki S, Tomi M, Hata T, Nagai Y, Hori S, Mori S, Hosoya K, Terasaki T (2005) Dominant expression of androgen receptors and their functional regulation of organic anion transporter 3 in rat brain capillary endothelial cells; comparison of gene expression between the blood-brain and -retinal barriers. J Cell Physiol 204:896–900. https://doi.org/10.1002/jcp.20352
Stirone C, Chu Y, Sunday L, Duckles SP, Krause DN (2003) 17 Beta-estradiol increases endothelial nitric oxide synthase mRNA copy number in cerebral blood vessels: quantification by real-time polymerase chain reaction. Eur J Pharmacol 478:35–38. https://doi.org/10.1016/j.ejphar.2003.08.037
Ospina JA, Duckles SP, Krause DN (2003) 17beta-estradiol decreases vascular tone in cerebral arteries by shifting COX-dependent vasoconstriction to vasodilation. Am J Physiol Heart Circ Physiol 285:H241-250. https://doi.org/10.1152/ajpheart.00018.2003
Bake S, Sohrabji F (2004) 17beta-estradiol differentially regulates blood-brain barrier permeability in young and aging female rats. Endocrinology 145:5471–5475. https://doi.org/10.1210/en.2004-0984
Stary JM, Wang BH, Moon SJ, Wang H (2014) Dramatic intracerebral hemorrhagic presentations of reversible cerebral vasoconstriction syndrome: three cases and a literature review. Case Rep Neurol Med 2014:782028. https://doi.org/10.1155/2014/782028
Singhal AB, Topcuoglu MA, Fok JW, Kursun O, Nogueira RG, Frosch MP, Caviness VS Jr (2016) Reversible cerebral vasoconstriction syndromes and primary angiitis of the central nervous system: clinical, imaging, and angiographic comparison. Ann Neurol 79:882–894. https://doi.org/10.1002/ana.24652
Topcuoglu MA, Singhal AB (2016) Hemorrhagic reversible cerebral vasoconstriction syndrome: features and mechanisms. Stroke 47:1742–1747. https://doi.org/10.1161/strokeaha.116.013136
Kastrup O, Schlamann M, Moenninghoff C, Forsting M, Goericke S (2015) Posterior reversible encephalopathy syndrome: the spectrum of MR imaging patterns. Clin Neuroradiol 25:161–171. https://doi.org/10.1007/s00062-014-0293-7
Lee WJ, Yeon JY, Jo KI, Kim JS, Hong SC (2015) Reversible cerebral vasoconstriction syndrome and posterior reversible encephalopathy syndrome presenting with deep intracerebral hemorrhage in young women. J Cerebrovasc Endovasc Neurosurg 17:239–245. https://doi.org/10.7461/jcen.2015.17.3.239
Forman R, Conners JJ, Song SY, John S, Garg R, Harris J, Lee VH (2019) The spectrum of nontraumatic convexity subarachnoid hemorrhage. J Stroke Cerebrovasc Dis 28:104473. https://doi.org/10.1016/j.jstrokecerebrovasdis.2019.104473
Muehlschlegel S, Kursun O, Topcuoglu MA, Fok J, Singhal AB (2013) Differentiating reversible cerebral vasoconstriction syndrome with subarachnoid hemorrhage from other causes of subarachnoid hemorrhage. JAMA Neurol 70:1254–1260. https://doi.org/10.1001/jamaneurol.2013.3484
Kumar S, Goddeau RP Jr, Selim MH, Thomas A, Schlaug G, Alhazzani A, Searls DE, Caplan LR (2010) Atraumatic convexal subarachnoid hemorrhage: clinical presentation, imaging patterns, and etiologies. Neurology 74:893–899. https://doi.org/10.1212/WNL.0b013e3181d55efa
Spitzer C, Mull M, Rohde V, Kosinski CM (2005) Non-traumatic cortical subarachnoid haemorrhage: diagnostic work-up and aetiological background. Neuroradiology 47:525–531. https://doi.org/10.1007/s00234-005-1384-6
Noda K, Fukae J, Fujishima K, Mori K, Urabe T, Hattori N, Okuma Y (2011) Reversible cerebral vasoconstriction syndrome presenting as subarachnoid hemorrhage, reversible posterior leukoencephalopathy, and cerebral infarction. Intern Med 50:1227–1233. https://doi.org/10.2169/internalmedicine.50.4812
Ansari SA, Rath TJ, Gandhi D (2011) Reversible cerebral vasoconstriction syndromes presenting with subarachnoid hemorrhage: a case series. J Neurointerv Surg 3:272–278. https://doi.org/10.1136/jnis.2010.004242
Nickele C, Muro K, Getch CC, Walker MT, Bernstein RA (2007) Severe reversible cerebral vasoconstriction syndrome mimicking aneurysmal rupture and vasospasm. Neurocrit Care 7:81–85. https://doi.org/10.1007/s12028-007-0001-4
Moustafa RR, Allen CM, Baron JC (2009) Call-Fleming syndrome associated with subarachnoid haemorrhage: three new cases. BMJ Case Rep. https://doi.org/10.1136/bcr.09.2008.0989
Berlit P, Kraemer M (2014) Cerebral vasculitis in adults: what are the steps in order to establish the diagnosis? Red flags and pitfalls. Clin Exp Immunol 175:419–424. https://doi.org/10.1111/cei.12221
Hajj-Ali RA, Singhal AB, Benseler S, Molloy E, Calabrese LH (2011) Primary angiitis of the CNS. Lancet Neurol 10:561–572. https://doi.org/10.1016/s1474-4422(11)70081-3
Hammad TA, Hajj-Ali RA (2013) Primary angiitis of the central nervous system and reversible cerebral vasoconstriction syndrome. Curr Atheroscler Rep 15:346. https://doi.org/10.1007/s11883-013-0346-4
Al-Mufti F, Dodson V, Wajswol E, El-Ghanem M, Alchaki A, Nuoman R, Thabet A, Sutherland A, Roychowdhury S, Hidalgo A, Gupta G (2018) Chemical angioplasty for medically refractory reversible cerebral vasoconstriction syndrome(). Br J Neurosurg 32:431–435. https://doi.org/10.1080/02688697.2018.1479512
Kraayvanger L, Berlit P, Albrecht P, Hartung HP, Kraemer M (2018) Cerebrospinal fluid findings in reversible cerebral vasoconstriction syndrome: a way to differentiate from cerebral vasculitis? Clin Exp Immunol 193:341–345. https://doi.org/10.1111/cei.13148
Zuber M, Touzé E, Domigo V, Trystram D, Lamy C, Mas JL (2006) Reversible cerebral angiopathy: efficacy of nimodipine. J Neurol 253:1585–1588. https://doi.org/10.1007/s00415-006-0269-x
Marsh EB, Ziai WC, Llinas RH (2016) The need for a rational approach to vasoconstrictive syndromes: transcranial doppler and calcium channel blockade in reversible cerebral vasoconstriction syndrome. Case Rep Neurol 8:161–171. https://doi.org/10.1159/000447626
Elstner M, Linn J, Müller-Schunk S, Straube A (2009) Reversible cerebral vasoconstriction syndrome: a complicated clinical course treated with intra-arterial application of nimodipine. Cephalalgia 29:677–682. https://doi.org/10.1111/j.1468-2982.2008.01768.x
John S, Singhal AB, Calabrese L, Uchino K, Hammad T, Tepper S, Stillman M, Mills B, Thankachan T, Hajj-Ali RA (2016) Long-term outcomes after reversible cerebral vasoconstriction syndrome. Cephalalgia 36:387–394. https://doi.org/10.1177/0333102415591507
Funding
Open Access funding enabled and organized by Projekt DEAL. No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
DKE: literature research, data analyzation, and drafting the manuscript. ACL: critical revision of the work. KA: conception and idea of the work, analyzation and interpretation of the data, critical revision of the manuscript, and final approval of the version to be published. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author declares that there is no conflict of financial or non-financial interest relevant to the content of this manuscript.
Ethics approval
This narrative review does not contain any identifying or protected data, which is why ethical approval was not obtained.
Consent to publish
Informed consent for publication of individual person’s data including any individual details and images was obtained from the patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Erhart, D.K., Ludolph, A.C. & Althaus, K. RCVS: by clinicians for clinicians—a narrative review. J Neurol 270, 673–688 (2023). https://doi.org/10.1007/s00415-022-11425-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-022-11425-z