Abstract
Objective
To investigate the prognostic value of white blood cell count (WBC) on functional outcome, mortality and bleeding risk in stroke patients treated with intravenous thrombolysis (IVT).
Methods
In this prospective multicenter study from the TRISP registry, we assessed the association between WBC on admission and 3-month poor outcome (modified Rankin Scale 3–6), mortality and occurrence of symptomatic intracranial hemorrhage (sICH; ECASS-II-criteria) in IVT-treated stroke patients. WBC was used as continuous and categorical variable distinguishing leukocytosis (WBC > 10 × 109/l) and leukopenia (WBC < 4 × 109/l). We calculated unadjusted/ adjusted odds ratios with 95% confidence intervals (OR [95% CI]) with logistic regression models. In a subgroup, we analyzed the association of combined leukocytosis and elevated C-reactive protein (CRP > 10 mg/l) on outcomes.
Results
Of 10,813 IVT-treated patients, 2527 had leukocytosis, 112 leukopenia and 8174 normal WBC. Increasing WBC (by 1 × 109/l) predicted poor outcome (ORadjusted 1.04[1.02–1.06]) but not mortality and sICH. Leukocytosis was independently associated with poor outcome (ORadjusted 1.48[1.29–1.69]) and mortality (ORadjusted 1.60[1.35–1.89]) but not with sICH (ORadjusted 1.17[0.94–1.45]). Leukopenia did not predict any outcome. In a subgroup, combined leukocytosis and elevated CRP had the strongest association with poor outcome (ORadjusted 2.26[1.76–2.91]) and mortality (ORadjusted 2.43[1.86–3.16]) when compared to combined normal WBC and CRP.
Conclusion
In IVT-treated patients, leukocytosis independently predicted poor functional outcome and death. Bleeding complications after IVT were not independently associated with leukocytosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic inflammation response is typical in patients with acute ischemic stroke (AIS) and can be caused by comorbidities (e.g., infections, malignancies or rheumatologic disorders) as well as local inflammatory processes of stroke-induced brain injury [1,2,3]. Inflammation enhances microvascular dysfunction and edema expansion, which ultimately can worsen the clinical outcome [4]. In clinical routine, white blood cell count (WBC) and C-reactive protein (CRP) are frequently used laboratory parameters to evaluate systemic inflammatory response [5].
In previous studies, leukocytosis (= elevated WBC) and elevated CRP were associated with more severe strokes and larger infarct volume in the general stroke population [6,7,8,9]. However, the effect of WBC and CRP on outcomes in acute stroke patients treated with IVT is contradictory. In one study, leukocytosis on admission was independently associated with poor functional outcome and occurrence of symptomatic intracranial hemorrhage (sICH) [10]. In contrast, another study did not find any association between baseline WBC and functional outcome, mortality or sICH [11]. Elevated CRP on admission was associated with poor functional outcome in one study [10] but not in another [6]. To the best of our knowledge, the combined effect of leukocytosis and elevated CRP on outcomes in IVT-treated stroke patients has not been investigated. In addition, the association of leukopenia with outcomes in this patient cohort is unknown.
With these considerations in mind, we conducted this multicenter cohort study to investigate, first, the association of WBC with outcomes in acute ischemic stroke patients treated with IVT. Second, we evaluated the prognostic importance of leukocytosis if combined with elevated CRP.
Methods
For this cohort study, we used prospectively collected data from the international, multicenter ThRombolysis in Ischemic Stroke Patients (TRISP) collaboration, which has been described previously [12]. Thirteen TRISP centers participated in this study (Table Supplementary-1). A complete list of all TRISP centers is presented in the appendix (Appendix Table 5). Data collection was done locally in each stroke center using a standardized form with predefined variables [13]. Data of the local registries were pooled and analyzed in an anonymized way at the stroke center Basel. Variables of interest for the present study were age, sex, National Institutes of Health Stroke Scale (NIHSS) score [14], blood pressure prior to IVT treatment, onset-to-treatment time, creatinine and glucose levels, WBC and CRP in blood samples on admission, vascular risk factors according to predefined criteria [15] and prior treatment with antithrombotic agents (antiplatelet agents or anticoagulants). Outcome parameters were mortality and the modified Rankin Scale (mRS) score at 3 months assessed by either outpatient visits or telephone calls with patients and/or relatives. Poor functional outcome was defined as a mRS score of 3–6. As safety outcome, we defined the occurrence of sICH using the ECASS-II-criteria [16]. Intracranial hemorrhage was monitored by follow-up CT or MRI as described in prior research [17].
Included data were collected between June 1995 and December 2018. We excluded patients with missing data on (i) WBC on admission or (ii) 3-month outcome.
Statistical analyses
Statistical analyses were performed with SPSS Statistics version 25 (IBM) and with R (R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/) and RStudio (RStudio Team (2022). RStudio: Integrated Development Environment for R. RStudio, PBC, Boston, MA URL http://www.rstudio.com/).
We investigated the associations between WBC and outcomes using WBC as a (i) continuous variable and as a (ii) categorical variable distinguishing leukocytosis (WBC > 10 × 109/l) and leukopenia (WBC < 4 × 109/l) according to current hematological definitions [18, 19]. Normal WBC (4–10 × 109/l) served as reference group.
Continuous data were summarized as median and interquartile range (IQR). We used chi2 test and Fisher’s exact test for categorical variables where appropriate and the Mann–Whitney U test for continuous variables. The association between WBC (± CRP) with each outcome was estimated by calculating odds ratios (OR) with 95% confidence intervals (95% CI), using binary logistic regression models. All baseline variables with Bonferroni adjusted p < 0.1 in univariable analyses were included in the multivariable analyses using stepwise regression with backward elimination based on the likelihood ratio test. To avoid overfitting, the maximum number of potential confounders in the final model was restricted to one-tenth of the number of outcome events, while maintaining age and NIHSS on admission as mandatory covariates in each model. Collinearity was checked in the final models by calculating the maximum generalized variance inflation factor using the car package in R, assuming a value below 5 indicating no relevant collinearity [20].
Subgroup analyses
In ten of the 13 participating centers, data on CRP on admission were available. We excluded patients from centers with missing data on CRP from the subgroup analysis (Fig. 1). In this subgroup, we assessed the association of combined leukocytosis and elevated CRP (normal range CRP < 10 mg/l [21]) on sICH, poor outcome and mortality. Patients with normal WBC and CRP served as reference group. In addition, we investigated the association between CRP and outcomes using CRP as a continuous variable.
Post hoc, we examined the association between leukocytosis and poor outcome and mortality using multivariable logistic regression models in patients admitted during three different time periods: 1995–2008, 2009–2015 and 2015–2018.
Standard protocol approvals, registrations, and patient consents
The study was approved by the ethics committee in Basel, Switzerland and written informed consent was waived. The requirement for additional local ethical approval differed between participating centers and was obtained if required.
Data availability statement
Anonymized data will be shared by request from any qualified investigator.
Results
Data were eligible for analysis in 10,813 (93.3%) of the 11,585 IVT-treated patients. Reasons for exclusion were missing data on WBC (n = 415; 3.6%) or 3-month mRS (n = 357; 3.1%) (Fig. 1).
Among eligible patients, 8174 (75.6%) had normal WBC, 2527 (23.4%) leukocytosis and 112 (1.0%) leukopenia.
Leukocytosis versus normal WBC
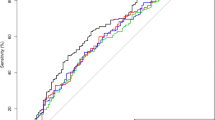
Baseline characteristics are presented in Table 1. Patients with leukocytosis were younger, more often active smokers, had more severe strokes on admission, longer-onset-to-treatment times and more often diabetes mellitus, prior ischemic stroke and pre-stroke disability compared to patients with normal WBC. Furthermore, patients with leukocytosis had more often poor functional outcome (49.7% vs 38.9%) and died more often (18.2% vs 11.8%) within the first 3 months and had a higher rate of sICH (5.1% vs 4.1%) (Table 1; Fig. 2).
In unadjusted logistic regression analyses, leukocytosis increased the odds of poor functional outcome (ORunadjusted 1.56, 95% CI 1.42–1.70), mortality (ORunadjusted 1.66, 95% CI 1.47–1.88) and sICH (ORunadjusted 1.26, 95% CI 1.02–1.55) (Table 2). After adjusting for potential confounders, the association of poor outcome (ORadjusted 1.48, 95% CI 1.29–1.69) and mortality (ORadjusted 1.60, 95% CI 1.35–1.89) with leukocytosis remained (Table 3). Using the same adjusted model, the association between leukocytosis and poor outcome and mortality remained stable over the long time period of data collection: (i) 1995–2008: poor outcome ORadjusted 1.44, 95% CI 1.10–1.89; mortality ORadjusted 1.57, 95% CI 1.12–2.19; (ii) 2009–2015: poor outcome ORadjusted 1.43, 95% CI 1.21–1.69; mortality 1.51, 95% CI 1.22–1.86; (iii) 2015–2018: poor outcome ORadjusted 2.07, 95% CI 1.33–3.21; mortality ORadjusted 2.17, 95% CI 1.35–3.48 (Table Supplementary-3). Leukocytosis increased the odds for sICH in the unadjusted (ORunadjusted 1.26, 95% CI 1.02–1.55) but not in the adjusted analyses (ORunadjusted 1.17, 95% CI 0.94–1.45) (Tables 2 and 3). The maximum variance inflation factor for this model was 1.19, suggesting no relevant collinearity disturbing the model.
Leukopenia versus normal WBC
Compared to normal WBC, leukopenia (n = 112; 1.04%) did not significantly change the odds for any outcome (poor functional outcome ORadjusted 1.05, 95% CI 0.66–1.65; mortality ORadjusted 1.32, 95% CI 0.69–2.38; sICH ORadjusted 1.02, 95% CI 0.35–2.30) (Table 3).
WBC as a continuous variable
Increasing WBC was associated with poor outcome (increase by 1 × 109/l, ORunadjusted 1.07, 95% CI 1.05–1.08) but not with mortality (ORunadjusted 1.00, 95% CI 1.00–1.01) and sICH (ORunadjusted 1.00, 95% CI 0.99–1.00) (Table 3). After adjusting for potential confounders the association of poor outcome (ORadjusted 1.04, 95% CI 1.02–1.06) with WBC remained. The maximum variance inflation factor for this model was 1.06, suggesting no relevant collinearity disturbing the model.
Subgroup of patients with available information on CRP and WBC
Out of 9651 patients, data on CRP were available in 8055 patients. (Fig. 1). Of those, 622 (7.7%) patients had elevated CRP combined with leukocytosis, whereas WBC and CRP were normal in 5′177 (64.3%) patients. Baseline characteristics of these two groups are presented in Table Supplementary-2. Poor outcome (64.0% vs 36.5%) and mortality (28.5% vs 10.3%) were more frequent in patients with combined leukocytosis and elevated CRP.
Combined leukocytosis and elevated CRP was independently associated with poor functional outcome (ORunadjusted 3.07, 95% CI 2.53–3.73 and ORadjusted 2.26, 95% CI 1.76–2.91) and mortality (ORunadjusted 3.86, 95% CI 3.09–4.81 and ORadjusted 2.43, 95% CI 1.86–3.16) but not with sICH compared to normal WBC and CRP (Table 4).
In the subgroup analysis, increasing CRP (by 1 mg/l) was associated with poor outcome (ORadjusted 1.01, 95% CI 1.01–1.02) and mortality (ORadjusted 1.01, 95% CI 1.01–1.02) but not with sICH (ORadjusted 1.00, 95% CI 0.99 -1.01) (Table 4).
Discussion
Key results for the association between white blood cell counts (WBC) and outcomes in acute ischemic stroke patients treated with IVT were: (i) Leukocytosis on admission independently predicted poor functional outcome and mortality. This association was even more pronounced in the subgroup of patients with combined leukocytosis and elevated CRP. (ii) Neither leukocytosis nor the combination of leukocytosis and elevated CRP was significantly associated with the occurrence of sICH. (iii) Leukopenia was not associated with any outcome.
Previous studies revealed an association between leukocytosis as well as elevated CRP on admission and more severe strokes and larger infarct volumes [6,7,8,9]. In line, patients with leukocytosis in the present study had higher baseline NIHSS than patients with normal WBC (median NIHSS 10 vs 8). This difference was even more pronounced in the subgroup of patients with combined leukocytosis and elevated CRP (median NIHSS 12 vs 8) suggesting that the extent of brain injury might — at least partly — be reflected by inflammatory parameters. Another explanation for the higher stroke severity in patients with elevated inflammatory parameters might be that those patients had significantly longer time intervals between stroke onset and administration than patients with normal WBC (median 166 min vs 140 min). Despite the fact, that our study was not designed to investigate potential causes of treatment delay, these associations stress the importance of investigating outcomes in this patient cohort. Yet, the effect of leukocytosis on outcomes and hemorrhagic complications in IVT-treated stroke patients in previous studies was controversial. One study, including 985 IVT-treated stroke patients, found an association between baseline leukocytosis and poor outcome [10], whereas in another study, including 846 IVT-treated stroke patients, baseline neutrophil count but not leukocytosis independently predicted poor outcome and mortality [11]. A third study found baseline WBC < 8.1 × 109/l to be associated with favorable outcome in IVT-treated stroke patients (n = 657) and suggested further investigation of WBC in larger prospective datasets [22]. Limitation of these studies is the relatively small number of patients with leukocytosis. In the present study, we found an independent association between leukocytosis and poor functional outcome and mortality, which remained stable over the long period of data collection (1995–2018). Interestingly, association with poor functional outcome and mortality was even stronger in patients with leukocytosis and elevated CRP. Additionally, the probability for poor functional outcome and mortality increased with rising WBC and CRP. However, every increase in WBC by 1 × 109/l increased the odds of poor functional outcome by 4% which is likely of minor clinical relevance. While we cannot evaluate the cause of the association between elevated inflammatory parameters and worse clinical outcome, it is likely that elevated inflammatory parameters contribute directly –e.g., via leucocyte clogging [23] — and indirectly — reflecting a co-existing disorder — to outcome after IVT.
Because patients with laboratory signs of systemic inflammation had higher NIHSS at onset and larger stroke volumes [6,7,8,9], it would have been likely that hemorrhagic complications after thrombolysis (sICH) occur more often in patients with laboratory signs of systemic inflammation than in those without. Accordingly, experimental research found that leukocytes contribute to vascular damage and disruption of the blood–brain barrier [24, 25]. Furthermore, one previous study revealed an independent association between baseline leukocytosis and occurrence of sICH [10] and another recent study investigating 510 ischemic stroke patients (196 treated with IVT) suggested an association of high WBC with parenchymal hemorrhage independent of infections [26]. However, in our study, after adjustment for potential confounders, neither leukocytosis nor the combination of leukocytosis and elevated CRP was independently associated with increased rate of sICH. Because increased baseline NIHSS, onset-to-treatment time and glucose levels on admission — all well-established risk factors of sICH — were more often present in patients with leukocytosis, we assume that the unadjusted association of leukocytosis with sICH is mostly due to these confounding parameters. Therefore, it seems unlikely that the association between elevated systemic inflammation parameters and poor outcome and mortality is caused by sICH.
Leukopenia — as defined by hematologic criteria — has not been investigated in IVT-treated stroke patients prior to this study. Numerous causes of leukopenia are likely to be associated with worse outcome (e.g., (viral) infections, (hematotoxic) drugs, autoimmune disorders, radiation, renal failure, malignancies, malnutrition) [27, 28]. Additionally, lymphopenia was shown to independently predict unfavorable outcome in intracerebral hemorrhage [29]. In our study, however, presence of leukopenia on admission was rare (1.0%) and did not change the odds for any outcome suggesting that leukopenia is rather a chance finding than a manifestation of an outcome modifying disorder in this patient cohort.
Strengths of our study are the large sample size (n = 10,813) which allowed adjusting for multiple confounding variables and thus clarifying the inconsistencies of previous studies. We also included a subgroup analysis with combined leukocytosis and elevated CRP which is a novelty and underlined the effect of systemic inflammation on clinical outcomes. Finally, we were able to assess outcomes in patients with leukopenia.
The present study has limitations apart from general limitations of register-based, retrospective studies: (i) causes of leukocytosis and elevated CRP were unclear and information on disorders that are likely associated with systemic inflammatory response (e.g., infections at admission, malignancies, rheumatologic disorders or certain medication) was not collected and it was not possible to determine whether leukocytosis was a trigger for stroke. However, as patients suffering from chronic infections or cancer are likely to have pre-existing disability, we were at least able to partly address this issue by adjusting for pre-stroke mRS. (ii) There is growing evidence on the strong linkage between ischemia and inflammation/ immunity [30, 31], and immunomodulation agents are evaluated for acute stroke therapy and stroke prevention [32, 33]. Yet, the observational design of our study does not allow conclusions on potential therapeutic measures modulating the inflammatory response. (iii) Leukocyte subclasses were not available. Thus, we were not able to examine the previously described association of neutrophils/ neutrophil-to-lymphocyte ratio with poor outcome, mortality and sICH [11] in our patient cohort. In addition, the association between leukopenia and outcomes was difficult to interpret as red blood cell and platelet counts were not available.
References
Jin R, Yang G, Li G (2010) Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol 87(5):779–789
Iadecola C, Anrather J (2011) The immunology of stroke: from mechanisms to translation. Nat Med 17:796–808
Planas AM (2018) Role of immune cells migrating to the ischemic brain. Stroke 49:2261–2267
De Meyer SF, Denorme F, Langhauser F, Geuss E, Fluri F, Kleinschnitz C (2016) Thromboinflammation in stroke brain damage. Stroke 47(4):1165–1172
Watt DG, Horgan PG, McMillan DC (2015) Routine clinical markers of the magnitude of the systemic inflammatory response after elective operation: a systematic review. Surgery 157(2):362–380
Rocco A, Ringleb PA, Grittner U, Nolte CH, Schneider A, Nagel S (2015) Follow-up C-reactive protein level is more strongly associated with outcome in stroke patients than admission levels. Neurol Sci 36(12):2235–2241
Kim J, Song TJ, Park JH et al (2012) Different prognostic value of white blood cell subtypes in patients with acute cerebral infarction. Atherosclerosis 222(2):464–467
Buck BH, Liebeskind DS, Saver JL et al (2008) Early neutrophilia is associated with volume of ischemic tissue in acute stroke. Stroke 39(2):355–360
den Hertog HM, van Rossum JA, van der Worp HB et al (2009) C-reactive protein in the very early phase of acute ischemic stroke: association with poor outcome and death. J Neurol 256(12):2003–2008
Tiainen M, Meretoja A, Strbian D et al (2013) Body temperature, blood infection parameters, and outcome of thrombolysis-treated ischemic stroke patients. Int J Stroke 8(8):632–638
Maestrini I, Strbian D, Gautier S et al (2015) Higher neutrophil counts before thrombolysis for cerebral ischemia predict worse outcomes. Neurology 85(16):1408–1416
Scheitz JF, Gensicke H, Zinkstok SM et al (2018) TRISP collaboration. Cohort profile: Thrombolysis in Ischemic Stroke Patients (TRISP): a multicentre research collaboration. BMJ Open 8:e023265
Engelter ST, Soinne L, Ringleb P et al (2011) IV thrombolysis and statins. Neurology 77:888–895
Lyden P, Brott T, Tilley B et al (1994) Improved reliability of the NIH Stroke Scale using video training. NINDS TPA Stroke Study Group. Stroke 25:2220–2226
Fluri F, Hatz F, Voss B, Lyrer PA, Engelter ST (2010) Restenosis after carotid endarterectomy: significance of newly acquired risk factors. Eur J Neurol 17:493–498
Hacke W, Kaste M, Fieschi C et al (1998) Randomised doubleblind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet 352:1245–1251
Gensicke H, Al Sultan AS, Strbian D et al (2018) Thrombolysis in stroke patients (TRISP) collaborators intravenous thrombolysis and platelet count. Neurology 90:e690–e697
Opdenakker G, Fibbe WE, Van Damme J (1998) The molecular basis of leukocytosis. Immunol Today 19(4):182–189
Meijer B, Kreijne JE, van Moorsel SAW et al (2017) 6-methylmercaptopurine-induced leukocytopenia during thiopurine therapy in inflammatory bowel disease patients. J Gastroenterol Hepatol 32(6):1183–1190
Fox J, Weisberg S (2019) An R Companion to Applied Regression, Third edition. Sage, Thousand Oaks CA. 2019. https://socialsciences.mcmaster.ca/jfox/Books/Companion/
Morley JJ, Kushner I (1982) Serum C-reactive protein levels in disease. Ann N Y Acad Sci 389:406–418
Malhotra K, Goyal N, Chang JJ et al (2018) Differential leukocyte counts on admission predict outcomes in patients with acute ischaemic stroke treated with intravenous thrombolysis. Eur J Neurol 25(12):1417–1424
El Amki M, Glück C, Binder N et al (2020) Neutrophils obstructing brain capillaries are a major cause of no-reflow in ischemic stroke. Cell Rep 33(2):108260
Rosell A, Cuadrado E, Ortega-Aznar A, Hernandez-Guillamon M, Lo EH, Montaner J (2008) MMP-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke 39:1121–1126
Jickling GC, Liu D, Ander BP, Stamova B, Zhan X, Sharp FR (2015) Targeting neutrophils in ischemic stroke: translational insights from experimental studies. J Cereb Blood Flow Metab 35(6):888–901
Semerano A, Strambo D, Martino G et al (2020) Leukocyte counts and ratios are predictive of stroke outcome and hemorrhagic complications independently of infections. Front Neurol 3(11):201
Newburger PE, Dale DC (2013) Evaluation and management of patients with isolated neutropenia. Semin Hematol 50(3):198–206
Warny M, Helby J, Nordestgaard BG, Birgens H, Bojesen SE (2018) Lymphopenia and risk of infection and infection-related death in 98,344 individuals from a prospective Danish population-based study. PLoS Med 15(11):e1002685
Giede-Jeppe A, Bobinger T, Gerner ST et al (2016) Lymphocytopenia is an independent predictor of unfavorable functional outcome in spontaneous intracerebral hemorrhage. Stroke 47(5):1239–1246
Perez-de-Puig I, Miro-Mur F, Ferrer-Ferrer M et al (2015) Neutrophil recruitment to the brain in mouse and human ischemic stroke. Acta Neuropathol 129:239–257
Lakhan SE, Kirchgessner A, Hofer M (2009) Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med 17(7):97
Malone K, Amu S, Moore AC, Waeber C (2019) Immunomodulatory therapeutic strategies in stroke. Front Pharmacol 10:630
Khandkar C, Vaidya K, Patel S (2019) Colchicine for stroke prevention: a systematic review and meta-analysis. Clin Ther 41(3):582-590.e3
Funding
Open access funding provided by University of Basel.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
V.L. Altersberger, G. Sibolt, A. Zietz, A. Schaufelbühl, A. Polymeris, C. Hametner, M. Heyse, G. Bigliardi, J. Stolp, T.J. van Velzen, G. Padlina, N. Slavova, M. Tiainen, K. Valkonen, L. Vandelli, M. Magoni, A. Luft, A. Pezzini, S. Nannoni, S. Räty, and S. Curtze report no disclosures. Lukas S Enz has received funding from the Swiss National Science Foundation (323530_171139). M.R. Heldner reports Scientific Advisory honoraria by Amgen and a grant from the Bangerter foundation, outside the submitted work. A. Zini has received funding for speaker honoraria and consulting fees from Boehringer-Ingelheim and Medtronic, speaker honoraria from Cerenovus, for scientific advisory board from Boehringer-Ingelheim and Stryker. V. Padjen travel or speaker honoraria from Boehringer Ingelheim and Pfizer; honoraria from scientific advisory board from Medtronic. C. Gumbinger is the head of the commission telestroke service of the German Stroke Society (DSG). D. Strambo has received congress travel support from Bristol-Myers Squibb, and research grant from the Swiss Heart Foundation and from the University of Lausanne. All fees are paid to his institution. C.W. Cereda has received modest honoraria for scientific advisory board from Bayer, Boehringer-Ingelheim and iSchemaview; Research grants from the Swiss Heart Foundation. Susanne Wegener received research funds by the Swiss National Science Foundation, the UZH Clinical research priority program (CRPP) stroke, the Swiss Heart foundation, Boehringer- Ingelheim, a speaker honorarium from Amgen and a consultancy fee from Bayer. Y. Béjot reports personal fees from AstraZeneca, BMS, Pfizer, Medtronic, MSD France, Amgen, Servier, and Boehringer-Ingelheim, outside the submitted work. D.R. Jovanović has received for travel or speaker honoraria from Bayer, Boehringer Ingelheim, Pfizer, Sanofi and Medtronic. She has served on scientific advisory board for Boehringer Ingelheim. P. Stanarcevic has received travel or speaker honoraria from Boehringer Ingelheim, Pfizer and Sandoz; also, honoraria from scientific advisory board from Medtronic and Boehringer Ingelheim. P. Michel has received has received through his institution research grants from the Swiss National Science Foundation, the Swiss Heart Foundation and the ERISTA program (Pfizer/BMS); and consulting fees from Medtronic. All this support is goes to his institution and is used for stroke education and research. P.A. Ringleb has received modest honoraria for lectures and advisory board from Boehringer-Ingelheim. The University Hospital Heidelberg is sponsor of the ECASS4-trial, examining the role of rtPA in an extended time-window, which is financed by Boehringer-Ingelheim. M. Arnold received Speaker honoraria from Bayer, Boehringer Ingelheim, and Covidien; Scientific advisory board honoraria from Amgen, Bayer, Boehringer Ingelheim, BMS, Pfizer, Covidien, Daichy Sankyo and Nestlé Health Science. Research grants from the Swiss Heart Foundation and the Swiss National Science Foundation. P.J. Nederkoorn has received funding from the Dutch heart foundation for acute stroke intervention trials in the Collaboration for New Trials in Stroke (CONTRAST) consortium. S.T. Engelter has received funding for travel or speaker honoraria from Bayer Boehringer-Ingelheim, and Daiichi-Sankyo. He has served on scientific advisory boards for Bayer, Boehringer-Ingelheim, BMS/Pfizer, MindMaze and on the editorial board of Stroke. He has received an educational grant from Pfizer and research support from the Science Funds (Wissenschaftsfonds) of the University Hospital Basel, the University Basel, the Swiss Heart Foundation and the Swiss National Science Foundation. H. Gensicke has received research support from the Swiss National Science Foundation, advisory board honoraria from Daiichi Sankyo and funding for travel from BMS/Pfizer.
Ethical standards
The study was approved by the ethics committee in Basel, Switzerland and written informed consent was waived. The requirement for additional local ethical approval differed between participating centers and was obtained if required.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Altersberger, V.L., Enz, L.S., Sibolt, G. et al. Thrombolysis in stroke patients with elevated inflammatory markers. J Neurol 269, 5405–5419 (2022). https://doi.org/10.1007/s00415-022-11173-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-022-11173-0