Abstract
Background
Psychiatric presentations similar to that observed in primary psychiatric disorders are well described across the amyotrophic lateral sclerosis–frontotemporal dementia (ALS–FTD) spectrum. Despite this, schizotypal personality traits associated with increased risks of clinical psychosis development and poor psychosocial outcomes have never been examined. The current study aimed to provide the first exploration of schizotypal traits and its neural underpinnings in the ALS–FTD spectrum to gain insights into a broader spectrum of psychiatric overlap with psychiatric disorders.
Methods
Schizotypal traits were assessed using the targeted Schizotypal Personality Questionnaire in 99 participants (35 behavioural variant FTD, 10 ALS–FTD and 37 ALS patients, and 17 age-, sex- and education-matched healthy controls). Voxel-based morphometry analysis of whole-brain grey matter volume was conducted.
Results
Relative to controls, pervasive schizotypal personality traits across positive and negative schizotypy and disorganised thought disorders were identified in behavioural variant FTD, ALS (with the exception of negative schizotypy) and ALS–FTDALS–FTD patients (all p < .013), suggesting the presence of a wide spectrum of subclinical schizotypal symptoms beyond classic psychotic symptoms. Atrophy in frontal, anterior cingulate and insular cortices, and caudate and thalamus was involved in positive schizotypy, while integrity of the cerebellum was associated with disorganised thought disorder traits.
Conclusions
The frontal–striatal–limbic regions underpinning manifestation of schizotypy in the ALS–FTDALS–FTD spectrum are similar to that established in previous schizophrenia research. This finding expands the concept of a psychiatric overlap in ALS–FTD and schizophrenia, and suggests potentially common underlying mechanisms involving disruptions to frontal-striatal-limbic networks, warranting a transdiagnostic approach for future investigations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS) can be considered as a continuum, with significant overlap across clinical, genetic and neuropathological features [1, 2]. Accumulating evidence suggests that psychosis can occur prior to the onset of typical cognitive, behavioural or motor symptoms in behavioural variant FTD (bvFTD) [3, 4] and ALS [5], as well as kindreds of ALS patients [6, 7]. Hallucinations and delusions, as classic positive symptoms of psychosis, have received the most research interest with a prevalence of up to 50% reported in patients with ALS–FTD and C9orf72 associated ALS–FTD [8,9,10]. Since its discovery in 2011, there has been mounting evidence of a differential clinical presentation in FTD and ALS associated with the C9orf72 repeat expansion characterised by a greater frequency of psychotic symptoms [11].
In addition to delusions and hallucinations, psychosis encompasses thought disorganisation and negative symptoms and that overlaps with the behavioural aspects of ALS–FTD. One study has examined the prevalence of all three subtypes of psychosis in bvFTD and found negative psychotic symptoms in 95.5% of bvFTD patients, followed by disorganised thought disorders (81.8%) and positive symptoms (22.7%)[12]. The prevalence of negative symptoms and disorganised thought have yet to be examined in ALS or ALS–FTD. While there is emerging evidence for more extensive overlap across ALS–FTD, C9orf72 and neuropsychiatric disorders, including schizophrenia and autism spectrum disorder (ASD) in terms of shared genetic susceptibility, brain network disruption and co-morbidities [5, 13], the nature of overlap in psychotic symptoms remains poorly understood due to the lack of studies directly comparing these disorders. As such, the use of questionnaires validated in the psychiatric populations tapping into all subdomains of psychosis represents an important first step towards characterising the psychiatric overlap with ALS–FTD spectrum.
However, the use of traditional clinical scales to measure clinical or manifesting psychosis in ALS–FTDALS–FTD precludes the detection of subclinical psychotic symptoms that have been associated with increased risks of clinical psychosis development and have a detrimental impact on well-being and functioning [14,15,16]. In particular, schizotypal personality traits represent a constellation of subclinical psychosis symptoms (e.g. magical thinking, ideas of reference and odd beliefs) that generally mirror those seen in schizophrenia yet are insufficient to warrant a clinical diagnosis. Evidence suggests that these traits may confer a risk of developing clinical psychosis [14,15,16] and are associated with psychiatric co-morbidities such as persistence of suicidal ideation [17], mood and substance use disorders [16], as well as functional impairment [16]. Exploration of schizotypal traits in the ALS–FTD spectrum may expand our understanding of individual susceptibility to psychosis and the wider spectrum of overlapping symptomatology across neuropsychiatric and neurodegenerative conditions.
Studies exploring the neural substrates of psychosis in bvFTD and ALS remain surprisingly scant. The few existing neuroimaging studies are limited by the lack of subtyping of psychotic symptoms with a primary focus on classic hallucination and delusion symptoms. Atrophy in an extensive network of regions involving bilateral prefrontal and occipital cortices, right thalamus and left cerebellum has been associated with greater severity of hallucinations and delusions across patients with bvFTD and ALS–FTD [18].
Extrapolating from the extensive psychosis literature available together with evidence from the ALS–FTD psychosis literature, we hypothesised that subclinical psychosis traits could be associated with a distributed region of atrophy that centres on the fronto-striatal–limbic network. As such, the present study aims to present a detailed exploration of (i) a broad spectrum of schizotypal personality traits across the ALS–FTD spectrum using a comprehensive measure of positive schizotypy, negative schizotypy, and thought disorganisation as a measure of psychosis risk; and (ii) the association between grey matter volume reduction and the severity of schizotypal traits within each domain using voxel-based morphometry (VBM) analysis to establish the neural correlates of schizotypy subtypes.
Materials and methods
Participants
In total, 99 participants (35 bvFTD, 10 ALS–FTD and 37 ALS patients, and 17 healthy controls) were recruited from the FRONTIER Clinic and the FOREFRONT MND and FTD Clinic, the clinical research clinics specialising in younger-onset dementias and motor neurodegenerative syndromes, respectively; based at the Brain and Mind Centre, University of Sydney, Australia. Standardised diagnostic assessment comprised of: a medical and neurological examination, neuropsychological assessment, clinical interviews and blood sampling. All ALS patients received further neurophysiological examination including nerve conduction studies and transcranial magnetic stimulation. Functional impairment in ALS and ALS–FTD patients was measured using the revised ALS functional rating scale (ALSFRS-R [19]). ALS and ALS–FTD patients with both limb and bulbar onset were included in the study. Cognitive testing was conducted by trained neurologists or neuropsychologists and motor impairments were taken into account. For patients who were unable to verbalise, they were given the opportunity to type or write their responses. Patients who were unable to communicate by any method were not included in the study. None of the patients required non-invasive ventilation at the time of study completion. For timed measure in terms of the fluency subdomain on the ACE-III, the verbal fluency index that controls for motor speed was used [20].
Diagnosis was determined by multidisciplinary consensus by a senior neurologist, clinical neurophysiologist and clinical neuropsychologist according to the current clinical diagnostic criteria for bvFTD [21], ALS–FTD [22] and ALS [23, 24]. All healthy participants were matched for age, sex and education years, and scored above the cutoff for normal range (> 88/100) on the Addenbrooke’s Cognitive Examination (ACE-III) [25]. All controls also underwent blood sampling to ensure absence of common ALS–FTD-related genetic mutations including C9orf72 repeat expansions, and mutation in the granulin (GRN) and microtubule-associated protein tau (MAPT) genes. Exclusion criteria for both patients and controls included the presence of other dementia syndrome, and/or pre-existing psychiatric disorders prior to disease onset.
Standard protocol approvals, registrations, and patient consents
Ethical approval was obtained from the South Eastern Sydney Local Health District, the University of New South Wales ethics committees and the University of Sydney ethics committees. All the participants or their person responsible provided written, informed consent to participate in accordance with the Declaration of Helsinki.
Blood sampling
All patients and controls underwent blood sampling to screen for a C9ORF72 repeat expansion, and GRN and MAPT mutations. Genomic DNA was extracted from peripheral blood lymphocytes. Proband DNA samples were then screened for the hexanucleotide repeat expansions in the C9ORF72 gene using a repeat primed polymerase chain reaction based on the protocol of Renton et al. [26] Samples were scored as positive if they harboured an allele with more than 30 repeats. Sanger sequencing was performed to identify mutations in GRN and MAPT.
Assessment of psychotic features
Detailed psychiatric evaluation was conducted with the carer where the presence of psychosis was determined based on the definition of delusion and hallucinations outlined in the Fifth edition of The Diagnostic and Statistical Manual of Mental Disorders (DSM-5) [27]. All interviews were conducted by a cognitive and behavioural neurologist (E.D.) with experiences in psychiatric features in ALS–FTD.
To further characterise the pattern and nature of schizotypal traits, all patients completed the well-validated 74-item Schizotypal Personality Questionnaire (SPQ) [28, 29] in a yes/no format (with a score of 1 assigned to each yes response). The higher the score, the more severe the schizotypal traits in the domains of positive schizotypy (i.e. magical ideation, unusual perceptual experiences referential thinking and suspiciousness), negative schizotypy (i.e. social anxiety, lack of close friends, constricted affect, and suspiciousness), and disorganised thought disorder (i.e. odd speech and odd behaviour). All items on the SPQ can be found in supplementary Table 1.
Statistical analysis
Data were analysed using SPSS Statistics, version 24.0 (IBM, Armonk, NY). The statistical significance level was set at p < 0.05 for all analyses unless otherwise specified. The assumption of normality was violated for most of the continuous variables based on Shapiro–Wilk results, therefore, non-parametric tests were used for all group comparisons unless otherwise specified. Differences in demographic (i.e., age and education) and SPQ subdomain scores (i.e. positive schizotypy, negative schizotypy and thought disorganisation) were examined between all groups using a Kruskal–Wallis test, followed by Mann–Whitney U post hoc comparisons with statistical significance set at a more conservative level of p < 0.013 for comparisons between disease groups (i.e., bvFTD, ALS–FTD, ALS and controls), and p < 0.017 for genetic status (i.e., C9orf72 carriers, non-carriers and controls) and psychosis status (i.e. patients identified as with and without positive psychosis on clinical interview, and controls), following Bonferroni correction for multiple comparisons (i.e., 0.05 divided by the number of levels of independent variables). Categorical variable (i.e. sex) was examined using chi-squared tests. Difference in clinical variables specific to ALS and ALS–FTD (i.e. ALSFRS-R score and site of onset) were also examined using independent sample t test and Chi-square test, respectively.
To examine whether C9orf72 results may be driven by particular diagnostic groups, the above analyses were repeated to compare demographic (i.e. sex, education years, age at scan), global cognitive (i.e., ACE total score), clinical (i.e., disease duration) and schizotypal (i.e. all three SPQ subdomain scores) characteristics between diagnostic subgroups with C9orf72 repeat expansions.
To account for the potential effect of motor impairment on the display of negative schizotypal traits (with an emphasis on social withdrawal) and disorganised thought disorder (characterised by odd speech and odd behaviour) in ALS and ALS–FTD, Spearman’s correlation was conducted to examine the correlation between ALSFRS-R score and negative schizotypy and disorganisation subdomain scores, respectively.
This approach allows for exploration of the nature and pattern of schizotypal traits associated with different diagnostic and genetic cohorts, as well as examination of subclinical schizotypal features in those that were traditionally considered to have psychotic symptoms defined as the presence of either hallucination or delusion according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria [27].
Imaging
Brain imaging acquisition
A subset of participants (33 bvFTD, 7 ALS–FTD, 29 ALS, and 17 controls) underwent a whole-brain structural MRI with a 3 T General Electric (GE) scanner, fitted with a standard eight-channel head coil. T1-weighted sequences were acquired using the following protocol: matrix = 256 × 256, 200 slices, 1mm2 in-plane resolution, slice thickness = 1 mm, echo time = 2.6 ms, repetition time = 5.8 ms, flip angle = 8°.
Imaging processing
All T1-weighted images were first visually inspected for the presence of any artefacts (e.g. head movements or poor contrast) by the investigators (N.Y.T and S.T.). As a result, ten ALS participants were excluded due to substantial head movement during image acquisition.
The final set of images (n = 76) were then analysed using VBM with Statistical Parametric Mapping version 12 (SPM12; Wellcome Department of Cognitive Neurology, London, UK) in Matlab R2018b (Mathworks, Natick, Massachusetts, USA) to examine whole-brain grey matter volumes. Firstly, T1-weighted images were segmented into five tissue probability maps in the native space. A Diffeomorphic Anatomical Registration using Exponentiated Lie (DARTEL) algebra template was then computed using the grey and white matter probability maps generated. Lastly, the grey matter probability maps were spatially normalised to the Montreal National Institute (MNI) space according to the transformation parameters from the DARTEL template. The resulting images were modulated and smoothed with a Gaussian kernel of 8 mm (full-width at half maximum).
Imaging statistical analysis
Firstly, whole-brain grey matter intensity differences between each patient group and controls were investigated using a voxel-wise whole-brain general linear model (GLM) with total intracranial volume included as a nuisance variable. The total intracranial volume was assessed in native space prior to spatial normalisation by summing the thresholded grey matter, white matter and corticospinal fluid probability maps (threshold = 0.2) and counting non-zero voxels.
Next, correlations between whole-brain grey matter volumes and each of the SPQ subdomains were examined using GLM covariate analyses across all patient groups. Specifically, the SPQ positive schizotypy, negative schizotypy, and thought disorganisation subdomain scores were entered as covariates in three separate GLMs with total intracranial volume as a nuisance variable to account for the effect of different head sizes. All analyses were corrected for cluster-extent multiple comparisons at p < 0.05, with a cluster-forming threshold of p < 0.001. All clusters were anatomically defined and visualised using xjView toolbox (http://www.alivelearn.net/xjview).
Results
Demographic characteristics
Comparisons between patient groups revealed significantly longer disease duration in bvFTD compared to both ALS (p < 0.001) and ALS–FTD (p = 0.002) groups, while ALS and ALS–FTD groups were not found to differ in disease duration, ALSFRS-R score or site of onset (p > 0.05; Table 1). No significant group differences were observed in sex, education or age across all groups (all p values > 0.05; Table 1). Both bvFTD and ALS–FTD groups demonstrated significantly lower ACE-III total scores compared to controls (both p < 0.001) and ALS patients (p < 0.001 and p = 0.003).
Comparisons between C9orf72 expansion carriers (n = 14), noncarriers (n = 68) and controls (n = 17) revealed no significant differences in age or education years (supplementary Table 2). Sex distribution, however, differed in the control group with a greater distribution of female participants (p = 0.016). Compared with controls, both C9orf72 carrier and noncarrier groups demonstrated significantly lower ACE-III total scores (both p < 0.001), while both patient groups demonstrated comparable ACE-III total scores (p = 0.151).
No significant differences were found in demographic or clinical variables (i.e. age, sex, education and disease duration; supplementary Table 3) across patients with and without psychosis, and controls. Patients with and without psychosis performed significantly worse on the ACE-III compared to controls (p < 0.001).
Psychotic features
ALS-FTD spectrum
Schizotypal features were evident across the ALS–FTD spectrum (Table 2). Relative to controls, both bvFTD and ALS–FTD groups demonstrated significantly higher positive schizotypy (p = 0.007; p < 0.001), negative schizotypy (p = 0.001; p < 0.001) and disorganisation (p = 0.004; p < 0.001) subdomain scores. Similarly, significantly higher positive schizotypy (p = 0.012) and thought disorganisation (p = 0.01) subdomain scores were observed in the ALS group. Significantly higher positive (p = 0.001) and negative (p = 0.010) schizotypy and disorganisation (p = 0.001) scores were further revealed in the ALS–FTD group compared to the ALS group.
In terms of the potential contribution of motor impairment to negative schizotypy and disorganisation expression, exploratory analyses did not reveal significant correlations between ALSFRS-R score and negative schizotypy or disorganisation subdomain scores (all p > 0.05; supplementary Table 4).
C9orf72 expansion carriers compared to noncarriers
Compared with controls, both C9orf72 expansion carriers and noncarriers demonstrated significantly higher SPQ scores across the positive schizotypy (p < 0.001; p = 0.008), negative schizotypy (p < 0.001; p = 0.003) and disorganisation (p < 0.001; p = 0.004) subdomains (Table 2). Disproportionately higher positive schizotypy scores were further revealed in C9orf72 carriers compared to noncarriers (p = 0.003).
Additional analyses did not reveal significant differences in demographic (i.e. sex, education years, age at scan; supplementary Table 5), global cognitive (i.e., ACE total score), clinical (i.e., disease duration) or schizotypal (i.e., all three SPQ subdomain scores) characteristics between diagnostic subgroups with C9orf72 repeat expansion, suggesting that the C9orf72 subanalysis results are unlikely to be driven by any of the diagnostic groups and representative of the ALS–FTD spectrum.
Patients presenting with positive psychosis compared to those without psychosis
Patients presenting with positive psychosis symptoms as determined by a traditional carer-based clinical interview displayed significantly higher scores across the positive schizotypy, negative schizotypy and disorganisation subdomains compared to those without positive psychosis and controls (all p values ≤ 0.001; Table 2). Furthermore, significantly higher negative schizotypy scores were revealed in patients without psychosis when compared to controls (p = 0.013).
VBM results
Widespread cortical grey matter intensity reduction that are largely consistent with previous reports in the literature was found in bvFTD (supplementary Fig. 1), ALS–FTD (supplementary Fig. 2) and ALS (supplementary Fig. 3) when compared to controls separately [30].
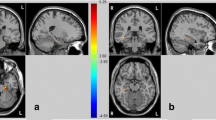
With all patient groups combined, positive schizotypy subdomain scores were found to be significantly associated with atrophy in bilateral superior and medial frontal gyrus, posterior cerebellum, and right anterior cingulate, insular cortex, inferior frontal gyrus, frontal orbital cortex, caudate and thalamus (Fig. 1 and Table 3). Significant correlations emerged between the cerebellum and disorganisation subdomain scores (Fig. 2 and Table 3). No significant correlation was identified within the negative schizotypy subdomain.
Brain regions that were significantly correlated with SPQ positive schizotypy subdomain scores for the ALS–FTD spectrum, controlling for total intracranial volume, using voxel-based morphometry analyses. Clusters were corrected for cluster-extent multiple comparisons at p < .05, with a cluster-forming threshold of p < .001. L left, R right
Brain regions that were significantly correlated with SPQ disorganised thought disorder subdomain scores across the ALS–FTD spectrum, controlling for total intracranial volume, using voxel-based morphometry analyses. Clusters were corrected for cluster-extent multiple comparisons at p < .05, with a cluster-forming threshold of p < .001. L left, R right
Discussion
Using a targeted measure of schizotypal traits and in combination with VBM analyses, we present the first comprehensive examination of a broad spectrum of subclinical psychosis symptoms in the ALS–FTD continuum, providing novel insights into the nature and pattern of schizotypy including the influence of genetic abnormalities on the manifestation of these symptoms with potential clinical and functional implications.
Capturing the full spectrum of schizotypal traits, higher expression of positive schizotypy relative to healthy controls were revealed in not only bvFTD and ALS–FTD, but also ALS patients, shedding light on the presence of positive schizotypal features in ALS, traditionally characterised by less severe behavioural disturbance. A higher frequency of positive schizotypy symptoms was found in C9orf72 compared to noncarriers, in line with previous findings of prominent neuropsychiatric presentation across bvFTD [11, 31, 32] and ALS [33] with C9orf72. These findings may have important clinical implications given the associated risk of progression into psychosis. In particular, positive schizotypal personality traits have been linked to the early prodromal stage of schizophrenia [34], representing a risk factor for schizophrenia [35, 36]. Therefore, clinical interview in conjunction with a targeted questionnaire (e.g. the SPQ) probing subtle psychotic-like features may offer avenues for detection of vulnerability to onset of frank psychosis.
Negative schizotypy was present in patients without frank positive psychosis, indicating that other subdomains of schizotypal features can present even in those without delusions or hallucinations. This is perhaps unsurprising given the overlap between the negative schizotypy and disorganisation symptoms and the behavioural (e.g. apathy) [21, 22] and motor features associated with the ALS–FTD spectrum, suggesting that psychiatric features overlap with behavioural features at the clinical level, likely as consequences of the disease process. Of further interest is the significant overlap in negative schizotypy symptoms implicated on the SPQ and hallmark features of ASD characterised by social withdrawal and difficulties with verbal and nonverbal communication in social interactions. The current findings consolidate the growing evidence of shared symptomatology between FTD and other neuropsychiatric conditions such as schizophrenia and ASD in addition to genetic susceptibility, brain network disruption and co-morbidities [5, 13].
Our neuroimaging analyses revealed that the breakdown of the fronto-striatal–limbic system plays a central role in the manifestation of positive schizotypy. Largely commensurate with the FTD literature [18], we identified a similar and wider network of regions including bilateral frontal, cerebellar regions, in addition to limbic and striatal structures including right anterior cingulate and insular cortices, caudate and thalamus in the manifestation of positive schizotypal traits. Fronto-striatal-limbic abnormalities have been extensively reported in schizophrenia research [37,38,39]. Abnormal striatal activation and disruption of fronto-striatal connectivity have previously been identified in individuals presenting with schizotypal personality traits [40], and unaffected first-degree relatives [41], respectively, supporting occurrence of neuropathological changes during the prodromal stage. Similar disruption of the fronto-striatal–limbic network in terms of grey matter volume loss in bilateral fronto-limbic regions (including anterior cingulate and insula) have been commonly found in both bvFTD and ALS [42], with more widespread frontal and limbic (including the thalamus) atrophy and additional striatal (including the caudate and putamen) atrophy further identified in bvFTD [43, 44]. Commonalities in the fronto-striatal–limbic abnormalities identified between ALS–FTD and schizophrenia suggests that the emergence of psychotic symptoms may be attributable to a similar underlying pathological mechanism. In other words, the regions implicated in schizophrenia may be applicable to the wider ALS–FTD population contributing to the development of shared psychotic experiences.
The posterior cerebellum was implicated in disorganised thought disorders. Cerebellum had traditionally been regarded as solely responsible for motor functions in coordination and execution of movement [45]. However, there has been robust evidence of cerebellar contribution to cognitive impairments in various clinical populations including all variants of FTD [46], ALS–FTD [47] and other syndromes of frontotemporal lobar degeneration [48]. The resulting cognitive and emotional disturbance are attributable to disruption of the connectivity between cerebellum and cortical areas subserving higher-order cognitive and emotion regulation functions [49, 50], leading to dysmetria of thought (i.e., discoordination of cognitive and emotional processing) analogous to discoordination of movements observed in cerebellar motor syndromes [51]. This suggests that cerebellar atrophy likely contributes to the emergence of higher-order thought disorganisation in ALS–FTD similar to that observed in schizophrenia, over and beyond its impact on motor functions.
While the current study provides the first detailed exploration of schizotypal traits across the ALS–FTD spectrum, several issues need to be considered. Firstly, the use of a self-report measure of schizotypal traits raises potential concern regarding the accuracy of self-evaluations. Given that current study is at the forefront of research on the potential overlap across psychiatric and neuropsychiatric conditions, our choice of measure is limited by the unavailability of an informant-based schizotypy measure that translates across psychiatric and neurodegenerative conditions. This is perhaps unsurprising given the challenges associated with detecting subtle psychosis-like internal states from a third-person perspective. The significant differences across all subdomains of schizotypy between patients classified as presenting with psychosis compared to those without through clinical interview conducted in the presence of a family member, however, supports the association between SPQ scores and clinically ascertained psychotic symptoms and provides supporting evidence of the sensitivity and validity of the SPQ. The use of a self-report measure is further supported by recent observations of a high level of correlation between patient- and carer-report versions of a psychosis measure in the ALS–FTD spectrum [52]. Nonetheless, the current findings remain an important first step towards establishing the utility of examining schizotypal symptoms in the ALS–FTD spectrum. Future efforts into developing a measure of schizotypal traits and psychotic features tailored to the FTD and/or ALS populations is warranted to facilitate its wider use in the clinical setting.
Further, the absence of significant association between brain structural changes and negative schizotypy may be related to the heavy focus on social factors on the SPQ negative psychosis subdomain where the majority of the questions concern social functioning (i.e. social communication difficulties, social anxiety and paranoid ideation) without tapping into classic negative psychotic symptoms such as loss of motivation and/or reduced initiation. This would appear to be in line with previous findings of a lack of interpersonal sensitivity and paranoid ideation in 111 ALS patients despite the presence of extensive psychological symptoms, including somatisation, anxiety, phobic anxiety, and depression [53]. In light of this, future studies will benefit from the additional use of a more comprehensive psychological measure that targets a wider dimension of psychological characteristics beyond the context of social functioning to comprehensively chart the neuropsychiatric profile across the ALS–FTD spectrum. Study of schizotypal features in pre-symptomatic carriers may provide insight into the time point that these features emerge and also determine whether they are a pre-requisite to frank psychosis in this population. Similarly, longitudinal assessment of structural brain changes beginning prior to onset of psychotic symptoms will help delineate the role of particular brain regions in the development of schizotypal traits, and subsequently, clinical psychosis at different disease stages. Lastly, future studies may consider the use of functional neuroimaging to examine functional connectivity in light of the increasing recognition that the manifestation of psychiatric disorders are generated from network disruption [54]. Moreover, this will also offer direct insight into whether different subtypes of schizotypal traits are underpinned by distinct functional networks.
In a similar vein, the absence of significant differences in negative schizotypy in the ALS group may be attributable to the relative preserved cognitive functioning in the current cohort of ALS patients given the recent finding of poorer cognitive performance in those with a personal or family history of neuropsychiatric disorders. It will therefore be beneficial for future studies with a larger sample of ALS patients to subclassify patients on the basis of presence of behavioural and/or cognitive impairment in accordance with the Strong et al. criteria [22] to assist in disentangling the relationship between the prevalence of subtypes of schizotypal symptoms and behavioural and cognitive deficits.
Conclusion
The nature of schizotypal traits in the ALS–FTD continuum is not limited to classic hallucinations or delusions with negative schizotypy and/or disorganised thought disorders evident across bvFTD, ALS–FTD and ALS. Current neuroimaging findings confirmed the involvement of fronto-striatal–limbic atrophy and cerebellar atrophy in the manifestation of positive psychosis and disorganised thoughts, respectively. This is in line with the frontal-striatal-limbic involvement reported extensively in previous schizophrenia research, suggesting that the shared psychotic features between the ALS–FTD spectrum and schizophrenia may be underpinned by disruption of a common network of brain regions. In addition to providing further insight into disease pathophysiology and better definition of clinical phenotype [55, 56], the present study raises further consideration of directed therapeutic interventions across these patient cohorts[57].
Data availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
References
Devenney E, Vucic S, Hodges JR, Kiernan MC (2015) Motor neuron disease-frontotemporal dementia: A clinical continuum. Expert Rev Neurother 15(5):509–522. https://doi.org/10.1586/14737175.2015.1034108
Burrell JR et al (2016) The frontotemporal dementia-motor neuron disease continuum. Lancet 388(10047):919–931. https://doi.org/10.1016/S0140-6736(16)00737-6
Scarioni M et al (2020) Frontotemporal dementia: correlations between psychiatric symptoms and pathology. Ann Neurol 87(6):950–961. https://doi.org/10.1002/ana.25739
Shinagawa S et al (2015) Clinicopathological study of patients with C9ORF72-associated frontotemporal dementia presenting with delusions. J Geriatr Psychiatry Neurol 28(2):99–107. https://doi.org/10.1177/0891988714554710
Zucchi E, Ticozzi N, Mandrioli J (2019) Psychiatric symptoms in amyotrophic lateral sclerosis: beyond a motor neuron disorder. Front Neurosci 13(March):1–11. https://doi.org/10.3389/fnins.2019.00175
Byrne S et al (2012) Cognitive and clinical characteristics of patients with amyotrophic lateral sclerosis carrying a C9orf72 repeat expansion: A population-based cohort study. Lancet Neurol 11(3):232–240. https://doi.org/10.1016/S1474-4422(12)70014-5
McLaughlin RL et al (2017) Genetic correlation between amyotrophic lateral sclerosis and schizophrenia. Nat Commun 8:1–12. https://doi.org/10.1038/ncomms14774
Hall D, Finger EC (2015) Psychotic Symptoms in Frontotemporal Dementia. Curr Neurol Neurosci Rep. https://doi.org/10.1007/s11910-015-0567-8
Silverman HE, Goldman JS, Huey ED (2019) Links between the C9orf72 repeat expansion and psychiatric symptoms. Curr Neurol Neurosci Rep. https://doi.org/10.1007/s11910-019-1017-9
Devenney E et al (2014) Frontotemporal dementia associated with the C9ORF72 mutation: a unique clinical profile. JAMA Neurol 71(3):331–339. https://doi.org/10.1001/jamaneurol.2013.6002
Rohrer JD et al (2015) C9orf72 expansions in frontotemporal dementia and amyotrophic lateral sclerosis. Lancet Neurol 14(3):291–301. https://doi.org/10.1016/S1474-4422(14)70233-9
Gossink FT et al (2017) Psychosis in behavioral variant frontotemporal dementia. Neuropsychiatr Dis Treat 13:1099–1106. https://doi.org/10.2147/NDT.S127863
Devenney EM, Ahmed RM, Halliday G, Piguet O, Kiernan MC, Hodges JR (2018) Psychiatric disorders in C9orf72 kindreds study of 1,414 family members. Neurology 91(16):E1498–E1507. https://doi.org/10.1212/WNL.0000000000006344
Walter EE, Fernandez F, Snelling M, Barkus E (2016) Genetic consideration of schizotypal traits: A review. Front Psychol. https://doi.org/10.3389/fpsyg.2016.01769
Debbané M, Eliez S, Badoud D, Conus P, Flückiger R, Schultze-Lutter F (2015) Developing psychosis and its risk states through the lens of schizotypy. Schizophr Bull 41(2):S396–S407. https://doi.org/10.1093/schbul/sbu176
Kwapil TR, Gross GM, Silvia PJ, Barrantes-Vidal N (2013) Prediction of psychopathology and functional impairment by positive and negative schizotypy in the chapmans’ ten-year longitudinal study. J Abnorm Psychol 122(3):807–815. https://doi.org/10.1037/a0033759
Kelleher I, Cederlöf M, Lichtenstein P (2014) Psychotic experiences as a predictor of the natural course of suicidal ideation: A Swedish cohort study. World Psychiatry 13(2):184–188. https://doi.org/10.1002/wps.20131
Devenney EM et al (2017) The neural correlates and clinical characteristics of psychosis in the frontotemporal dementia continuum and the C9orf72 expansion. NeuroImage Clin 13:439–445. https://doi.org/10.1016/j.nicl.2016.11.028
Cedarbaum JM et al (1999) The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci 169(1–2):13–21. https://doi.org/10.1016/S0022-510X(99)00210-5
Abrahams S et al (1997) Relation between cognitive dysfunction and pseudobulbar palsy in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 62(5):464–472. https://doi.org/10.1136/jnnp.62.5.464
Rascovsky K et al. (2011) Behavioural variant of frontotemporal dementia. https://doi.org/10.1093/brain/awr179
Strong MJ et al (2017) Amyotrophic lateral sclerosis - frontotemporal spectrum disorder (ALS-FTSD): Revised diagnostic criteria. Amyotroph Lateral Scler Front Degener 18(3–4):153–174. https://doi.org/10.1080/21678421.2016.1267768
de Carvalho M et al (2008) Electrodiagnostic criteria for diagnosis of ALS. Clin Neurophysiol 119(3):497–503. https://doi.org/10.1016/j.clinph.2007.09.143
Shefner JM et al (2020) A proposal for new diagnostic criteria for ALS. Clin Neurophysiol 131(8):1975–1978. https://doi.org/10.1016/j.clinph.2020.04.005
Hsieh S, Schubert S, Hoon C, Mioshi E, Hodges JR (2013) Validation of the Addenbrooke’s cognitive examination III in frontotemporal dementia and Alzheimer’s disease. Dement Geriatr Cogn Disord 36(3–4):242–250. https://doi.org/10.1159/000351671
Renton AE et al (2011) A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS–FTD. Neuron 72(2):257–268. https://doi.org/10.1016/j.neuron.2011.09.010
American Psychiatric Association (2013) Highlights of changes from DSM-IV to DSM-5,” Diagnostic Stat Man Ment Disord 11(4), 525–527 https://doi.org/10.1176/appi.books.9780890425596.changes
Fonseca-Pedrero E et al (2018) The structure of schizotypal personality traits: A cross-national study. Psychol Med 48(3):451–462. https://doi.org/10.1017/S0033291717001829
Raine A (1991) The spq: A scale for the assessment of schizotypal personality based on DSM-III-r criteria. Schizophr Bull 17(4):555–564. https://doi.org/10.1093/schbul/17.4.555
Ahmed RM et al (2021) Tackling clinical heterogeneity across the amyotrophic lateral sclerosis–frontotemporal dementia spectrum using a transdiagnostic approach. Brain Commun 3(4):1–14. https://doi.org/10.1093/braincomms/fcab257
Liu Y, Yu JT, Sun FR, Ou JR, Ben Qu S, Tan L (2013) “The clinical and pathological phenotypes of frontotemporal dementia with C9ORF72 mutations. J Neurol Sci 335(1–2):26–35. https://doi.org/10.1016/j.jns.2013.09.013
Woollacott IOC, Mead S (2014) The C9ORF72 expansion mutation: Gene structure, phenotypic and diagnostic issues. Acta Neuropathol 127(3):319–332. https://doi.org/10.1007/s00401-014-1253-7
De Souza PVS, Pinto WBVDR, Oliveira ASB (2015) Distúrbios relacionados ao C9orf72: Expandindo o espectro clínico e genético das doenças neurodegenerativas. Arq Neuropsiquiatr 73(3):246–256. https://doi.org/10.1590/0004-282X20140229
Schultze-Lutter F, Nenadic I, Grant P (2019) Psychosis and schizophrenia-spectrum personality disorders require early detection on different symptom dimensions. Front Psychiatry. https://doi.org/10.3389/fpsyt.2019.00476
Vollema MG, Sitskoorn MM, Appels MCM, Kahn RS (2002) Does the Schizotypal Personality Questionnaire reflect the biological-genetic vulnerability to schizophrenia? Schizophr Res 54(1–2):39–45. https://doi.org/10.1016/S0920-9964(01)00350-4
Torgersen S et al (2002) Schizotypal personality disorder inside and outside the schizophrenic spectrum. Schizophr Res 54(1–2):33–38. https://doi.org/10.1016/S0920-9964(01)00349-8
Dietsche B, Kircher T, Falkenberg I (2017) Structural brain changes in schizophrenia at different stages of the illness: A selective review of longitudinal magnetic resonance imaging studies. Aust N Z J Psychiatry 51(5):500–508. https://doi.org/10.1177/0004867417699473
Hu ML et al (2017) A review of the functional and anatomical default mode network in schizophrenia. Neurosci Bull 33(1):73–84. https://doi.org/10.1007/s12264-016-0090-1
Birur B, Kraguljac NV, Shelton RC, Lahti AC (2017) Brain structure, function, and neurochemistry in schizophrenia and bipolar disorder- A systematic review of the magnetic resonance neuroimaging literature. Npj Schizophr 3(1):1–14. https://doi.org/10.1038/s41537-017-0013-9
Kirschner M et al (2018) Ventral striatal dysfunction and symptom expression in individuals with schizotypal personality traits and early psychosis. Schizophr Bull 44(1):147–157. https://doi.org/10.1093/schbul/sbw142
Fornito A et al (2013) Functional dysconnectivity of corticostriatal circuitry as a risk phenotype for psychosis. JAMA Psychiat 70(11):1143–1151. https://doi.org/10.1001/jamapsychiatry.2013.1976
Luo C, Hu N, Xiao Y, Zhang W, Gong Q, Lui S (2020) Comparison of gray matter atrophy in behavioral variant frontal temporal dementia and amyotrophic lateral sclerosis: a coordinate-based meta-analysis. Front Aging Neurosci 12(February):1–12. https://doi.org/10.3389/fnagi.2020.00014
Pan PL et al (2012) Gray matter atrophy in behavioral variant frontotemporal dementia: A meta-analysis of voxel-based morphometry studies. Dement Geriatr Cogn Disord 33(2–3):141–148. https://doi.org/10.1159/000338176
Pan PL, Liu Y, Zhang Y, Zhao H, Ye X, Xu Y (2017) Brain gray matter abnormalities in progressive supranuclear palsy revisited. Oncotarget 8(46):80941–80955. https://doi.org/10.18632/oncotarget.20895
Holmes G (1939) The cerebellum of man. Brain 62(1):1–30. https://doi.org/10.1093/brain/62.1.1
Chen Y, Kumfor F, Landin-Romero R, Irish M, Hodges JR, Piguet O (2018) Cerebellar atrophy and its contribution to cognition in frontotemporal dementias. Ann Neurol 84(1):98–109. https://doi.org/10.1002/ana.25271
Tan RH, Devenney E, Kiernan MC, Halliday GM, Hodges JR, Hornberger M (2015) Terra incognita-cerebellar contributions to neuropsychiatric and cognitive dysfunction in behavioral variant frontotemporal dementia. Front Aging Neurosci 7:1–9. https://doi.org/10.3389/fnagi.2015.00121
Tse NY et al (2020) Cerebellar contributions to cognition in corticobasal syndrome and progressive supranuclear palsy. Brain Commun 2(2):1–13. https://doi.org/10.1093/braincomms/fcaa194
Stoodley CJ (2012) The cerebellum and cognition : evidence from functional imaging studies. The Cerebellum. https://doi.org/10.1007/s12311-011-0260-7
Chen Y, Landin-Romero R, Kumfor F, Irish M, Hodges JR, Piguet O (2020) Cerebellar structural connectivity and contributions to cognition in frontotemporal dementias. Cortex 129:57–67. https://doi.org/10.1016/j.cortex.2020.04.013
Schmahmann JD (1998) Dysmetria of thought: Clinical consequences of cerebellar dysfunction on cognition and affect. Trends Cogn Sci 2(9):362–371. https://doi.org/10.1016/S1364-6613(98)01218-2
Devenney EM et al (2021) Neural mechanisms of psychosis vulnerability and perceptual abnormalities in the ALS–FTD spectrum. Ann Clin Transl Neurol 8(8):1576–1591. https://doi.org/10.1002/acn3.51363
Felgoise SH et al (2010) Psychological morbidity in ALS: The importance of psychological assessment beyond depression alone. Amyotroph Lateral Scler 11(4):351–358
Fornito A, Bullmore ET, Zalesky A (2017) Opportunities and Challenges for Psychiatry in the Connectomic Era. Biol Psychiatry Cogn Neurosci Neuroimaging 2(1):9–19. https://doi.org/10.1016/j.bpsc.2016.08.003
Kiernan MC et al (2011) Amyotrophic lateral sclerosis. Lancet 377(9769):942–955. https://doi.org/10.1016/S0140-6736(10)61156-7
Turner MR et al (2013) Controversies and priorities in amyotrophic lateral sclerosis. Lancet Neurol 12(3):310–322. https://doi.org/10.1016/S1474-4422(13)70036-X
Kiernan MC et al (2021) Improving clinical trial outcomes in amyotrophic lateral sclerosis. Nat Rev Neurol 17(2):104–118. https://doi.org/10.1038/s41582-020-00434-z
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This study was funded by the National Health and Medical Research Council, 1037746, 1132524, 1095127, APP1121859, Sicong Tu, Post-doctoral fellowship, Rebekah M. Ahmed, 1176607, Glenda M. Halliday, APP1103258, Olivier Piguet, Centre of Excellence in Cognition and its Disorders, Australian Research Council, CE110001021, Motor Neurone Disease Research Institute of Australia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors report no conflict interests.
Ethical approval
All the participants or their person responsible provided written, informed consent to participate in accordance with the Declaration of Helsinki.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tse, N.Y., Tu, S., Chen, Y. et al. Schizotypal traits across the amyotrophic lateral sclerosis–frontotemporal dementia spectrum: pathomechanistic insights. J Neurol 269, 4241–4252 (2022). https://doi.org/10.1007/s00415-022-11049-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-022-11049-3