Abstract
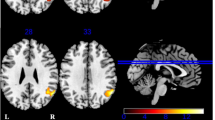
Patients with Parkinson’s disease (PD) have difficulties processing action words, which could be related to early cognitive decline. The action fluency test can be used to quickly and easily assess the processing of action words in PD. The goal of this study was to characterize how the action fluency test relates to personal characteristics, disease factors, cognition, and neural activity in PD. Forty-eight participants with PD (34 male, 14 female) and 35 control participants (16 male, 19 female) completed functional neuroimaging using a set-shifting task and a neuropsychological assessment including the action fluency test. PD participants with a score one standard deviation below the norm or lower on the action fluency test were identified. All PD participants with poor performance (PD-P, n = 15) were male. They were compared to male PD participants with scores within the normal range (PD-N, n = 19) and male healthy controls (HC, n = 16). PD-P were older, had lower global cognition scores, lower executive functions scores, and decreased activity in fronto-temporal regions compared with PD-N. There was no difference between the two PD groups in terms of the duration of the disease, dose of dopaminergic medication, and severity of motor symptoms. PD-N were younger than HC, but there was no other significant difference between these groups. The action fluency test identified a subgroup of PD patients with distinct sex, age, global cognition, executive functions, and brain activity characteristics. Implications for the evaluation of cognition are discussed.


Similar content being viewed by others
Availability of data and material
Data are available from the senior or corresponding author upon request.
References
Weil RS, Costantini AA, Schrag AE (2018) Mild Cognitive Impairment in Parkinson's Disease-What Is It? Curr Neurol Neurosci Rep 18(4):17. https://doi.org/10.1007/s11910-018-0823-9
Goldman JG, Vernaleo BA, Camicioli R, Dahodwala N, Dobkin RD, Ellis T, Galvin JE, Marras C, Edwards J, Fields J, Golden R, Karlawish J, Levin B, Shulman L, Smith G, Tangney C, Thomas CA, Troster AI, Uc EY, Coyan N, Ellman C, Ellman M, Hoffman C, Hoffman S, Simmonds D (2018) Cognitive impairment in Parkinson's disease: a report from a multidisciplinary symposium on unmet needs and future directions to maintain cognitive health. NPJ Parkinsons Dis 4:19. https://doi.org/10.1038/s41531-018-0055-3
Todorova A, Jenner P, Chaudhuri RK (2014) Non-motor Parkinson's: integral to motor Parkinson's, yet often neglected. Pract Neurol 14(5):310–322. https://doi.org/10.1136/practneurol-2013-000741
Litvan I, Aarsland D, Adler CH, Goldman JG, Kulisevsky J, Mollenhauer B, Rodriguez-Oroz MC, Troster AI, Weintraub D (2011) MDS Task Force on mild cognitive impairment in Parkinson's disease: critical review of PD-MCI. Mov Disord 26(10):1814–1824. https://doi.org/10.1002/mds.23823
Litvan I, Goldman JG, Troster AI, Schmand BA, Weintraub D, Petersen RC, Mollenhauer B, Adler CH, Marder K, Williams-Gray CH, Aarsland D, Kulisevsky J, Rodriguez-Oroz MC, Burn DJ, Barker RA, Emre M (2012) Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov Disord 27(3):349–356. https://doi.org/10.1002/mds.24893
Piatt AL, Fields JA, Paolo AM, Koller WC, Tröster AI (1999) Lexical, semantic, and action verbal fluency in Parkinson's disease with and without dementia. J Clin Exp Neuropsychol 21(4):435–443. https://doi.org/10.1076/jcen.21.4.435.885
Caramazza A, Hillis AE (1991) Lexical organization of nouns and verbs in the brain.pdf. Nature 349:788–790
Damasio AR, Tranel D (1993) Nouns and verbs are retrieved with differently distributed neural systems. Proc Natl Acad Sci USA 90:4957–4960
Grossman M (1998) Not all words are created equal: Category-specific deficits in central nervous system disease. Neurology 50(2):324–325. https://doi.org/10.1212/wnl.50.2.324
Piatt AL, Fields JA, Paolo AM, Tröster AI (1999) Action (naming) fluency as an executive function measure: convergent and divergent evidence of validity. Neuropsychologia 37:1499–1503
Piatt AL, Fields JA, Paolo AM, Tröster AI (2004) Action verbal fluency normative data for the elderly. Brain Lang 89(3):580–583. https://doi.org/10.1016/j.bandl.2004.02.003
Woods SP, Scott JC, Sires DA, Grant I, Heaton RK, Tröster AI, Center THNR (2005) Action (verb) fluency: test-retest reliability, normative standards and construct validity. J Int Neuropsychol Soc 11:408–415
Signorini M, Volpato C (2006) Action fluency in Parkinson's disease: a follow-up study. Mov Disord 21(4):467–472. https://doi.org/10.1002/mds.20718
Druks J, Masterson J, Kopelman M, Clare L, Rose A, Rai G (2006) Is action naming better preserved (than object naming) in Alzheimer's disease and why should we ask? Brain Lang 98(3):332–340. https://doi.org/10.1016/j.bandl.2006.06.003
Vigliocco G, Vinson DP, Druks J, Barber H, Cappa SF (2011) Nouns and verbs in the brain: a review of behavioural, electrophysiological, neuropsychological and imaging studies. Neurosci Biobehav Rev 35(3):407–426. https://doi.org/10.1016/j.neubiorev.2010.04.007
Kim M, Thompson CK (2004) Verb deficits in Alzheimer’s disease and agrammatism: implications for lexical organization. Brain Lang 88(1):1–20. https://doi.org/10.1016/s0093-934x(03)00147-0
Szekely A, Damico S, Devescovi A, Federmeier K, Herron D, Iyer G, Jacobsen T, Arevalo A, Vargha A, Bates E (2005) Timed action and object naming. Cortex 41(1):7–25. https://doi.org/10.1016/s0010-9452(08)70174-6
Thompson CK, Lukic S, King MC, Mesulam MM, Weintraub S (2012) Verb and noun deficits in stroke-induced and primary progressive aphasia: The Northwestern Naming Battery(). Aphasiology 26(5):632–655. https://doi.org/10.1080/02687038.2012.676852
Auclair-Ouellet N, Lieberman P, Monchi O (2017) Contribution of language studies to the understanding of cognitive impairment and its progression over time in Parkinson's disease. Neurosci Biobehav Rev 80:657–672. https://doi.org/10.1016/j.neubiorev.2017.07.014
Cardona JF, Gershanik O, Gelormini-Lezama C, Houck AL, Cardona S, Kargieman L, Trujillo N, Arevalo A, Amoruso L, Manes F, Ibanez A (2013) Action-verb processing in Parkinson's disease: new pathways for motor-language coupling. Brain Struct Funct 218(6):1355–1373. https://doi.org/10.1007/s00429-013-0510-1
Cardona JF, Kargieman L, Sinay V, Gershanik O, Gelormini C, Amoruso L, Roca M, Pineda D, Trujillo N, Michon M, Garcia AM, Szenkman D, Bekinschtein T, Manes F, Ibanez A (2014) How embodied is action language? Neurological evidence from motor diseases. Cognition 131(2):311–322. https://doi.org/10.1016/j.cognition.2014.02.001
Auclair-Ouellet N, Fossard M, Macoir J, Laforce R Jr (2020) The nonverbal processing of actions is an area of relative strength in the semantic variant of primary progressive aphasia. J Speech Lang Hear Res 63(2):569–584. https://doi.org/10.1044/2019_JSLHR-19-00271
Coslett HB, Saffran EM, Schwoebel J (2002) Knowledge of the human body: a distinct semantic domain. Neurology 59:357–363
Martin A, Chao LL (2001) Semantic memory and the brain: structure and processes. Curr Opin Neurobiol 11:194–201
Pulvermüller F, Hauk O, Nikulin VV, Ilmoniemi RJ (2005) Functional links between motor and language systems. Eur J Neurosci 21(3):793–797. https://doi.org/10.1111/j.1460-9568.2005.03900.x
Meteyard L, Cuadrado SR, Bahrami B, Vigliocco G (2012) Coming of age: a review of embodiment and the neuroscience of semantics. Cortex 48(7):788–804. https://doi.org/10.1016/j.cortex.2010.11.002
Mahon BZ, Caramazza A (2008) A critical look at the embodied cognition hypothesis and a new proposal for grounding conceptual content. J Physiol Paris 102(1–3):59–70. https://doi.org/10.1016/j.jphysparis.2008.03.004
Mahon BZ (2015) What is embodied about cognition? Lang Cogn Neurosci 30(4):420–429. https://doi.org/10.1080/23273798.2014.987791
Watson CE, Cardillo ER, Ianni GR, Chatterjee A (2013) Action concepts in the brain: an activation likelihood estimation meta-analysis. J Cogn Neurosci 25(8):1191–1205. https://doi.org/10.1162/jocn_a_00401
Courson M, Macoir J, Tremblay P (2017) Role of medial premotor areas in action language processing in relation to motor skills. Cortex 95:77–91. https://doi.org/10.1016/j.cortex.2017.08.002
Postle N, McMahon KL, Ashton R, Meredith M, de Zubicaray GI (2008) Action word meaning representations in cytoarchitectonically defined primary and premotor cortices. Neuroimage 43(3):634–644. https://doi.org/10.1016/j.neuroimage.2008.08.006
Pulvermüller F (2018) Neural reuse of action perception circuits for language, concepts and communication. Prog Neurobiol 160:1–44. https://doi.org/10.1016/j.pneurobio.2017.07.001
Pulvermüller F (2005) Brain mechanisms linking language and action. Nat Rev Neurosci 6:576–582
Binder JR, Desai RH (2011) The neurobiology of semantic memory. Trends Cogn Sci 15(11):527–536. https://doi.org/10.1016/j.tics.2011.10.001
Binder JR, Desai RH, Graves WW, Conant LL (2009) Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex 19(12):2767–2796. https://doi.org/10.1093/cercor/bhp055
Patterson K, Nestor PJ, Rogers TT (2007) Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci 8(12):976–987. https://doi.org/10.1038/nrn2277
Lambon Ralph MA, Jefferies E, Patterson K, Rogers TT (2017) The neural and computational bases of semantic cognition. Nat Rev Neurosci 18(1):42–55. https://doi.org/10.1038/nrn.2016.150
Bookheimer S (2002) Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci 25:151–188. https://doi.org/10.1146/annurev.neuro.25.112701.142946
Monchi O, Petrides M, Petre V, Worsley K, Dagher A (2001) Wisconsin Card Sorting Revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. J Neurosci 21:7733–7741
Nagano-Saito A, Leyton M, Monchi O, Goldberg YK, He Y, Dagher A (2008) Dopamine depletion impairs frontostriatal functional connectivity during a set-shifting task. J Neurosci 28(14):3697–3706. https://doi.org/10.1523/JNEUROSCI.3921-07.2008
Monchi O, Petrides M, Doyon J, Postuma RB, Worsley K, Dagher A (2004) Neural bases of set-shifting deficits in Parkinson's disease. J Neurosci 24(3):702–710. https://doi.org/10.1523/JNEUROSCI.4860-03.2004
Nagano-Saito A, Habak C, Mejia-Constain B, Degroot C, Monetta L, Jubault T, Bedetti C, Lafontaine AL, Chouinard S, Soland V, Ptito A, Strafella AP, Monchi O (2014) Effect of mild cognitive impairment on the patterns of neural activity in early Parkinson's disease. Neurobiol Aging 35(1):223–231. https://doi.org/10.1016/j.neurobiolaging.2013.06.025
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Nasreddine ZS, Phillipps NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005) The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53:695–699
Heaton RK, Miller W, Taylor MJ, Grant I (2004) Revised comprehensive norms for an expanded Halsted-Reitan battery: demographically adjusted neuropsychological norms for African American and Caucasian adults—Professional manual. Psychological Assessment Resources, Lutz
Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012) FSL. Neuroimage 62(2):782–790. https://doi.org/10.1016/j.neuroimage.2011.09.015
Smith SM (2002) Fast robust automated brain extraction. Hum Brain Mapp 17(3):143–155. https://doi.org/10.1002/hbm.10062
Jenkinson M, Bannister P, Brady M, Smith S (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage 17(2):825–841. https://doi.org/10.1006/nimg.2002.1132
Fengler S, Roeske S, Heber I, Reetz K, Schulz JB, Riedel O, Wittchen HU, Storch A, Linse K, Baudrexel S, Hilker R, Mollenhauer B, Witt K, Schmidt N, Balzer-Geldsetzer M, Dams J, Dodel R, Graber S, Pilotto A, Petrelli A, Funkele S, Kassubek J, Kalbe E (2016) Verbal memory declines more in female patients with Parkinson's disease: the importance of gender-corrected normative data. Psychol Med 46(11):2275–2286. https://doi.org/10.1017/S0033291716000908
Miller IN, Cronin-Golomb A (2010) Gender differences in Parkinson's disease: clinical characteristics and cognition. Mov Disord 25(16):2695–2703. https://doi.org/10.1002/mds.23388
Picillo M, Nicoletti A, Fetoni V, Garavaglia B, Barone P, Pellecchia MT (2017) The relevance of gender in Parkinson's disease: a review. J Neurol 264(8):1583–1607. https://doi.org/10.1007/s00415-016-8384-9
Vegeto E, Villa A, Della Torre S, Crippa V, Rusmini P, Cristofani R, Galbiati M, Maggi A, Poletti A (2020) The role of sex and sex hormones in neurodegenerative diseases. Endocr Rev. https://doi.org/10.1210/endrev/bnz005
Aarsland D, Bronnick K, Williams-Gray C, Weintraub D, Marder K, Kulisevsky J, Burn D, Barone P, Pagonabarraga J, Allcock L, Santangelo G, Foltynie T, Janvin C, Larsen JP, Barker RA, Emre M (2010) Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology 75(12):1062–1069. https://doi.org/10.1212/WNL.0b013e3181f39d0e
Nagano-Saito A, Bellec P, Hanganu A, Jobert S, Mejia-Constain B, Degroot C, Lafontaine AL, Lissemore JI, Smart K, Benkelfat C, Monchi O (2019) Why Is aging a risk factor for cognitive impairment in Parkinson's disease?—a resting state fMRI Study. Front Neurol 10:267. https://doi.org/10.3389/fneur.2019.00267
Lin SJ, Baumeister TR, Garg S, McKeown MJ (2018) Cognitive Profiles and Hub Vulnerability in Parkinson's Disease. Front Neurol 9:482. https://doi.org/10.3389/fneur.2018.00482
Crossley NA, Mechelli A, Scott J, Carletti F, Fox PT, McGuire P, Bullmore ET (2014) The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain 137(Pt 8):2382–2395. https://doi.org/10.1093/brain/awu132
Baggio HC, Sala-Llonch R, Segura B, Marti MJ, Valldeoriola F, Compta Y, Tolosa E, Junque C (2014) Functional brain networks and cognitive deficits in Parkinson's disease. Hum Brain Mapp 35(9):4620–4634. https://doi.org/10.1002/hbm.22499
Alexander GE, DeLong MR, Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci 9(1):357–381. https://doi.org/10.1146/annurev.ne.09.030186.002041
Mathys C, Hoffstaedter F, Caspers J, Caspers S, Sudmeyer M, Grefkes C, Eickhoff SB, Langner R (2014) An age-related shift of resting-state functional connectivity of the subthalamic nucleus: a potential mechanism for compensating motor performance decline in older adults. Front Aging Neurosci 6:178. https://doi.org/10.3389/fnagi.2014.00178
Ystad M, Hodneland E, Adolfsdottir S, Haasz J, Lundervold AJ, Eichele T, Lundervold A (2011) Cortico-striatal connectivity and cognition in normal aging: a combined DTI and resting state fMRI study. Neuroimage 55(1):24–31. https://doi.org/10.1016/j.neuroimage.2010.11.016
Jahanshahi M, Obeso I, Rothwell JC, Obeso JA (2015) A fronto-striato-subthalamic-pallidal network for goal-directed and habitual inhibition. Nat Rev Neurosci 16(12):719–732. https://doi.org/10.1038/nrn4038
Whiteside DM, Kealey T, Semla M, Luu H, Rice L, Basso MR, Roper B (2016) Verbal Fluency: Language or Executive Function Measure? Appl Neuropsychol Adult 23(1):29–34. https://doi.org/10.1080/23279095.2015.1004574
Smith KM, Caplan DN (2018) Communication impairment in Parkinson's disease: Impact of motor and cognitive symptoms on speech and language. Brain Lang 185:38–46. https://doi.org/10.1016/j.bandl.2018.08.002
Munro CA, Winicki JM, Schretlen DJ, Gower EW, Turano KA, Munoz B, Keay L, Bandeen-Roche K, West SK (2012) Sex differences in cognition in healthy elderly individuals. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 19(6):759–768. https://doi.org/10.1080/13825585.2012.690366
Kløve H (1963) Clinical neuropsychology. In: Forster FM (ed) The medical clinics of North America. Saunders, New York
Williams-Gray CH, Evans JR, Goris A, Foltynie T, Ban M, Robbins TW, Brayne C, Kolachana BS, Weinberger DR, Sawcer SJ, Barker RA (2009) The distinct cognitive syndromes of Parkinson's disease: 5 year follow-up of the CamPaIGN cohort. Brain 132(Pt 11):2958–2969. https://doi.org/10.1093/brain/awp245
Association AP (1994) Diagnostic and statistical manual of mental disorders, 4th edn. Association AP, Washington DC
Auclair-Ouellet N, Mandl S, Kibreab M, Haffenden A, Hanganu A, Cheetham J, Kathol I, Sarna J, Martino D, Monchi O (2020) Characterization of cognition in mild cognitive impairment with and without Parkinson's disease. Clin Parkinsonism Relat Disord. https://doi.org/10.1016/j.prdoa.2020.100034
Cholerton BA, Zabetian CP, Wan JY, Montine TJ, Quinn JF, Mata IF, Chung KA, Peterson A, Espay AJ, Revilla FJ, Devoto J, Watson GS, Hu SC, Leverenz JB, Edwards KL (2014) Evaluation of mild cognitive impairment subtypes in Parkinson's disease. Mov Disord 29(6):756–764. https://doi.org/10.1002/mds.25875
Das T, Hwang JJ, Poston KL (2019) Episodic recognition memory and the hippocampus in Parkinson's disease: A review. Cortex 113:191–209. https://doi.org/10.1016/j.cortex.2018.11.021
Davey J, Rueschemeyer SA, Costigan A, Murphy N, Krieger-Redwood K, Hallam G, Jefferies E (2015) Shared neural processes support semantic control and action understanding. Brain Lang 142:24–35. https://doi.org/10.1016/j.bandl.2015.01.002
Davey J, Thompson HE, Hallam G, Karapanagiotidis T, Murphy C, De Caso I, Krieger-Redwood K, Bernhardt BC, Smallwood J, Jefferies E (2016) Exploring the role of the posterior middle temporal gyrus in semantic cognition: integration of anterior temporal lobe with executive processes. Neuroimage 137:165–177. https://doi.org/10.1016/j.neuroimage.2016.05.051
Bak TH, Hodges JR (2003) Kissing and dancing—a test to distinguish the lexical and conceptual contributions to noun/verb and action/object dissociation. Preliminary results in patients with frontotemporal dementia. J Neurolinguist 16:169–181
Funding
This work was supported by a Canadian Institutes of Health Research (CIHR) Fellowship to N.A.O. (grant number FAH-381468), a postdoctoral fellowship from Parkinson Alberta to A.H., a project grant from the CIHR (grant number PJT-166123), the Canada Research Chair in non-motor deficits of Parkinson’s disease, and the Tourmaline Oil Chair in Parkinson's Disease to O.M. N.A.O. and A.H. are supported by Research Scholar Junior 1 Salary Awards from the Fonds de Recherche en Santé du Québec (FRQS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest/Competing interests
None to declare.
Consent to participate
The project was conducted according to principles stated in the declaration of Helsinki and all the participants provided written informed consent to participate in this study.
Consent for publication
All authors have read the final version of the manuscript and have agreed to be included in the list of authors.
Ethics approval
The project was approved by the Research Ethics Board of the University of Calgary.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Auclair-Ouellet, N., Hanganu, A., Mazerolle, E.L. et al. Action fluency identifies different sex, age, global cognition, executive function and brain activation profile in non-demented patients with Parkinson’s disease. J Neurol 268, 1036–1049 (2021). https://doi.org/10.1007/s00415-020-10245-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-10245-3