Abstract
Background
MRI is highly sensitive for monitoring of disease activity and treatment efficacy in MS. Patients treated with disease modifying therapy (DMT), who experience MRI activity, including contrast-enhancing lesions (CEL) or new/enlarged T2 lesions, should be evaluated for a switch to more effective treatment. Due to recent evidence of gadolinium (Gd) accumulation in the brain after repeated administration of Gd-based contrast agents, FDA recommended to limit its use.
Aim
To investigate the proportion of cases in which MRI activity would be detectable only using contrast-enhanced T1-weighted sequences.Secondary aims were to assess the presence of clinical or demographic variables associated with reactivation of pre-existing lesions and to analyse therapeutic consequences of different types of MRI lesions.
Methods
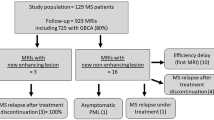
We retrospectively evaluated brain MRI scans, performed between 2014 and 2018, in patients treated with DMT for at least 6 months.
Results
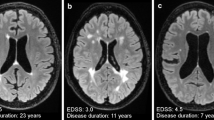
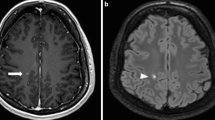
We analysed 906 scans in 255 patients. New/enlarged T2 lesions were detected in 13.7% of cases, CEL in 3.5%, CEL without new T2 lesions (old lesions reactivated) in 1.1%. No variables were associated with old lesions reactivated. CEL with T2 equivalent were at higher risk of DMT switch, compared with new/enlarged T2 lesions without corresponding CEL (OR 4.0, 95% CI 1.5–10.4, p = 0.005).
Conclusions
Reactivation of pre-existing lesions is limited to a tiny fraction of MRI studies. Gd + T1-weighted images could be omitted, in patients treated with DMT for at least 6 months, without relevant loss of information.

Similar content being viewed by others
Availability of data and material
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Rovira A, Wattjes MP, Tintoré M, Tur C, Yousry TA et al (2015) MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis—clinical implementation in the diagnostic process. Nat Rev Neurol 11:471–482
Wattjes MP, Rovira A, Miller D, Yousry TA, Sormani MP, De Stefano N et al (2015) MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis—establishing disease prognosis and monitoring patients. Nat Rev Neurol 11:597–606
Rio J, Castillo J, Rovira A, Tintoré M, Sastre-Garriga J, Horga A et al (2009) Measures in the first year of therapy predict the response to interferon beta in MS. Mult Scler 15(7):848–853
Sormani MP, Stubinski B, Cornelisse P, Rocak S, Li D, De Stefano N (2011) Magnetic resonance active lesions as individual-level surrogate for relapses in multiple sclerosis. Mult Scler 17(5):541–549
Bermel RA, You X, Foulds P, Hyde R, Simon JH, Fisher E, Rudick RA (2013) Predictors of long-term outcome in multiple sclerosis patients treated with interferon beta. Ann Neurol 73:95–103
Miller DH, Barkhof F, Nauta JJ (1993) Gadolinium enhancement increases the sensitivity of MRI in detecting disease activity in multiple sclerosis. Brain 116(Pt 5):1077–1094
Filippi M, Rovaris M, Bastianello S, Gasperini C, Origgi D, Reganati P et al (1999) A comparison of the sensitivity of monthly unenhanced and enhanced MRI techniques in detecting new multiple sclerosis lesions. J Neurol 246(2):97–106
Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D (2014) High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 270:834–841
Errante Y, Cirimele V, Mallio CA, Di Lazzaro V, Zobel BB, Quattrocchi CC (2014) Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Invest Radiol 49:685–690
Weberling LD, Kieslich PJ, Kickingereder P, Wick W, Bendszus M, Schlemmer HP, Radbruch A (2015) Increased signal intensity in the dentate nucleus on unenhanced T1-weighted images after gadobenate dimeglumine administration. Invest Radiol 50:743–748
Adin ME, Kleinberg L, Vaidya D, Zan E, Mirbagheri S, Yousem DM (2015) Hyperintense dentate nuclei on T1-weighted MRI: relation to repeat gadolinium administration. AJNR Am J Neuroradiol 36:1859–1865
U.S. Food and Drug Administration (2015) FDA drug safety communication: FDA evaluating the risk of brain deposits with repeated use of gadolinium-based contrast agents for magnetic resonance imaging (MRI). Safety announcement. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-evaluating-risk-brain-deposits-repeated-use-gadolinium-based. Accessed 27 July 2015
U.S. Food and Drug Administration (2017) FDA drug safety communication: FDA identifies no harmful effects to date with brain retention of gadolinium-based contrast agents for MRIs; review to continue. Safety announcement. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-identifies-no-harmful-effects-date-brain-retention-gadolinium#collapseOne. Accessed 22 May 2017
European Medicines Agency (2017) EMA’s final opinion confirms restrictions on use of linear gadolinium agents in body scans, pp 1–4. Available at: https://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2017/07/news_detail_002780.jsp&mid=WC0b01ac058004d5c1. Accessed 23 Nov 2017
Eichinger P, Schon S, Pongratz V, Wiestler H, Zhang H, Bussas M et al (2019) Accuracy of unenhanced MRI in the detection of new brain lesions in multiple sclerosis. Radiology 291:429–435
Rudie JD, Mattay RR, Schindler M, Steingalla S, Cook TS, Loevner LA et al (2019) An initiative to reduce unnecessary gadolinium-based contrast in multiple sclerosis patients. J Am Coll Radiol 16:1158–1164
Karimian-Jazi K, Wildemann B, Diem R, Schwarz D, Hielscher T, Wick W et al (2018) Gd contrast administration is dispensable in patients with MS without new T2 lesions on follow-up MRI. Neurol Neuroimmunol Neuroinflamm 5:e480
Mattay RR, Davtyan K, Bilello M, Mamourian AC (2018) Do all patients with multiple sclerosis benefit from the use of contrast on serial follow-up MR imaging? A retrospective analysis. AJNR Am J Neuroradiol 39:2001–2006
Sadigh G, Saindane AM, Waldman AD, Lava NS, Hu R (2019) Comparison of unenhanced and gadolinium-enhanced imaging in multiple sclerosis: is contrast needed for routine follow-up MRI? AJNR Am J Neuroradiol 40(9):1476–1480
Johnston G, Johnson T, Bazylewicz M, Solomon A, Ulano A (2019) Gadolinium contrast may be unnecessary when evaluating for interval disease activity in patients with multiple sclerosis. Mult Scler 25(S2):131–356
Gentili L, Gallo A, Gaetani L, Capuano R, Bisecco A, D'Ambrosio A et al (2019) Evidence of disease activity in active multiple sclerosis is gadolinium-based contrast mandatory? Mult Scler 25(S2):357–580
Battaglini M, Rossi F, Grove RA, Stromillo ML, Whitcher B, Matthews PM, De Stefano N (2014) Automated identification of brain new lesions in multiple sclerosis using subtraction images. J Magn Reson Imaging 39:1543–1549
Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G et al (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 17(2):162–173
Lublin FD, Reingold SC, Cohen JA, Cutter GR, Soelberg Sørensen P, Thompson AJ et al (2014) Defining the clinical course of multiple sclerosis. Neurology 83:1–9
Swanton JK, Fernando KT, Dalton CM, Miszkiel KA, Altmann DR, Plant GT et al (2009) Early MRI in optic neuritis: the risk for disability. Neurology 72:542–550
Okuda DT, Mowry EM, Beheshtian A, Waubant E, Baranzini SE, Goodin DS et al (2009) Incidental MRI anomalies suggestive of multiple sclerosis: the radiologically isolated syndrome. Neurology 72(9):800–805
Cotton F, Weiner HL, Jolesz FA, Guttmann CR (2003) MRI contrast uptake in new lesions in relapsing-remitting MS followed at weekly intervals. Neurology 60:640–646
Gulani V, Calamante F, Shellock FG, Kanal E, Reeder SB (2017) International society for magnetic resonance in medicine. Magnetic resonance in gadolinium deposition in the brain: summary of evidence and recommendations. Lancet Neurol 16:564–570
Zivadinov R, Bergsland N, Hagemeier J, Ramasamy DP, Dwyer MG, Schweser F et al (2019) Cumulative gadodiamide administration leads to brain gadolinium deposition in early MS. Neurology 93:e611–e623
Brisset JC, Kremer S, Hannoun S, Bonneville F, Durand-Dubief F, Tourdias T, Barillot C et al (2020) New OFSEP recommendations for MRI assessment of multiple sclerosis patients: special consideration for gadolinium deposition and frequent acquisitions. J Neuroradiol. https://doi.org/10.1016/j.neurad.2020.01.083
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
E. Tsantes served on scientific advisory boards for Roche, Almirall and Merck & Co; has received funding for travel from Biogen, Merck & Co, Sanofi Genzyme, Roche, Novartis and Mylan. E. Curti served on scientific advisory boards for Merck & Co and Novartis; has received funding for travel from Biogen, Merck & Co, Teva Pharmaceutical Industries, Sanofi Genzyme, Roche and Novartis. C. Ganazzoli has nothing to disclose. F. Puci has nothing to disclose. V. Bazzurri has received funding for travel from Sanofi Genzyme, Biogen and Roche. A. Fiore has received funding for travel from Sanofi Genzyme and Biogen. G. Crisi has nothing to disclose. F. Granella has received research grants for his Institution from Biogen and Sanofi Genzyme; has served on scientific advisory boards for Biogen, Novartis, Sanofi Genzyme, Roche and Merck Serono; and has received funding for travel from Biogen, Merck Serono and Sanofi Genzyme.
Ethical statement
The study was approved by the local ethics committee (Comitato Etico Area Vasta Emilia Nord, 484/2018/OSS/AOUPR) and performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
All the patients provided written informed consent.
Rights and permissions
About this article
Cite this article
Tsantes, E., Curti, E., Ganazzoli, C. et al. The contribution of enhancing lesions in monitoring multiple sclerosis treatment: is gadolinium always necessary?. J Neurol 267, 2642–2647 (2020). https://doi.org/10.1007/s00415-020-09894-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-09894-1