Abstract
Introduction
Treatment with botulinum toxin A is the evidence-based first-line therapy of cervical dystonia. The aim of this study was to analyze long-term data of the most commonly used products concerning safety and efficacy in a big cohort over decades.
Methods
We retrospectively analyzed the treatment data of all cervical dystonia patients in our outpatient clinic having at least three treatment sessions with current onabotulinumtoxinA or abobotulinumtoxinA. The observation period was up to 17 years for onabotulinumtoxinA and 27 years for abobotulinumtoxinA. We report on safety and efficacy, comparing parameters such as dose, treatment intervals, side effects, occurrence and reasons of primary or secondary non-response.
Results
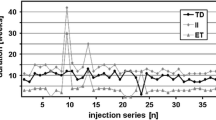
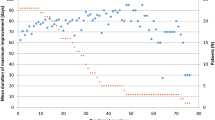
We analyzed a total of 2592 and 6660 treatment sessions in 135 patients with onabotulinumtoxinA, 209 with abobotulinumtoxinA and 10 having received both preparations. We found a moderate increase of dosage in the first years which was succeeded by a stable mean dose (138 and 663 mouse units, respectively) and stable injection intervals from the beginning. The most frequent side effects were mild dysphagia (2/6%), muscle weakness (2/6%) and pain (2/2%). Reasons for therapy discontinuation were change to a nearby doctor, age, other diseases, spontaneous improvement, side effects or possible treatment failure. Of all patients, only two tested positive for neutralizing antibodies against botulinum toxin A.
Conclusion
We show that treatment of cervical dystonia with the two most frequently used botulinum toxin A preparations is a safe and effective therapy even over a long treatment duration of up to 27 years.





Similar content being viewed by others
References
Norris SA, Jinnah HA, Espay AJ, Klein C, Bruggemann N, Barbano RL, Malaty IA, Rodriguez RL, Vidailhet M, Roze E, Reich SG, Berman BD, LeDoux MS, Richardson SP, Agarwal P, Mari Z, Ondo WG, Shih LC, Fox SH, Berardelli A, Testa CM, Cheng FC, Truong D, Nahab FB, Xie T, Hallett M, Rosen AR, Wright LJ, Perlmutter JS (2016) Clinical and demographic characteristics related to onset site and spread of cervical dystonia. Mov Disord. https://doi.org/10.1002/mds.26817
Albanese A, Bhatia K, Bressman SB, Delong MR, Fahn S, Fung VS, Hallett M, Jankovic J, Jinnah HA, Klein C, Lang AE, Mink JW, Teller JK (2013) Phenomenology and classification of dystonia: a consensus update. Mov Disord 28(7):863–873. https://doi.org/10.1002/mds.25475
Leplow B, Eggebrecht A, Pohl J (2017) Treatment satisfaction with botulinum toxin: a comparison between blepharospasm and cervical dystonia. Patient Prefer Adher 11:1555–1563. https://doi.org/10.2147/PPA.S141060
Gill CE, Manus ND, Pelster MW, Cook JA, Title W, Molinari AL, Charles D (2013) Continuation of long-term care for cervical dystonia at an academic movement disorders clinic. Toxins (Basel) 5(4):776–783. https://doi.org/10.3390/toxins5040776
Haussermann P, Marczoch S, Klinger C, Landgrebe M, Conrad B, Ceballos-Baumann A (2004) Long-term follow-up of cervical dystonia patients treated with botulinum toxin A. Mov Disord 19(3):303–308. https://doi.org/10.1002/mds.10659
Skogseid IM, Kerty E (2005) The course of cervical dystonia and patient satisfaction with long-term botulinum toxin A treatment. Eur J Neurol 12(3):163–170. https://doi.org/10.1111/j.1468-1331.2004.01053.x
Ramirez-Castaneda J, Jankovic J (2014) Long-term efficacy, safety, and side effect profile of botulinum toxin in dystonia: a 20-year follow-up. Toxicon 90:344–348. https://doi.org/10.1016/j.toxicon.2014.07.009
Vivancos-Matellano F, Ybot-Gorrin I, Diez-Tejedor E (2012) A 17-year experience of abobotulinumtoxina in cervical dystonia. Int J Neurosci 122(7):354–357. https://doi.org/10.3109/00207454.2012.665971
Brin MF, Comella CL, Jankovic J, Lai F, Naumann M, Group CDBS (2008) Long-term treatment with botulinum toxin type A in cervical dystonia has low immunogenicity by mouse protection assay. Mov Disord 23(10):1353–1360. https://doi.org/10.1002/mds.22157
Bentivoglio AR, Di Stasio E, Mulas D, Cerbarano ML, Ialongo T, Laurienzo A, Petracca M (2017) Long-term abobotulinumtoxin A treatment of cervical dystonia. Neurotox Res 32(2):291–300. https://doi.org/10.1007/s12640-017-9737-6
Kessler KR, Skutta M, Benecke R (1999) Long-term treatment of cervical dystonia with botulinum toxin A: efficacy, safety, and antibody frequency. German Dystonia Study Group. J Neurol 246(4):265–274
Hefter H, Rosenthal D, Moll M (2016) High botulinum toxin-neutralizing antibody prevalence under long-term cervical dystonia treatment. Mov Disord Clin Pract 3(5):500–506. https://doi.org/10.1002/mdc3.12322
Jog M, Wein T, Bhogal M, Dhani S, Miller R, Ismail F, Beauchamp R, Trentin G (2016) Real-world, long-term quality of life following therapeutic OnabotulinumtoxinA treatment. Can J Neurol Sci 43(5):687–696. https://doi.org/10.1017/cjn.2016.262
Mejia NI, Vuong KD, Jankovic J (2005) Long-term botulinum toxin efficacy, safety, and immunogenicity. Mov Disord 20(5):592–597. https://doi.org/10.1002/mds.20376
Dressler D, Tacik P, Saberi FA (2015) Botulinum toxin therapy of cervical dystonia: duration of therapeutic effects. J Neural Transm (Vienna) 122(2):297–300. https://doi.org/10.1007/s00702-014-1253-8
Vogt T, Lussi F, Paul A, Urban P (2008) Long-term therapy of focal dystonia and facial hemispasm with botulinum toxin A. Nervenarzt 79(8):912–917. https://doi.org/10.1007/s00115-008-2486-2
Mohammadi B, Buhr N, Bigalke H, Krampfl K, Dengler R, Kollewe K (2009) A long-term follow-up of botulinum toxin A in cervical dystonia. Neurol Res 31(5):463–466. https://doi.org/10.1179/174313209X405137
Ferreira JJ, Colosimo C, Bhidayasiri R, Marti MJ, Maisonobe P, Om S (2015) Factors influencing secondary non-response to botulinum toxin type A injections in cervical dystonia. Parkinsonism Relat Disord 21(2):111–115. https://doi.org/10.1016/j.parkreldis.2014.09.034
Jankovic J, Vuong KD, Ahsan J (2003) Comparison of efficacy and immunogenicity of original versus current botulinum toxin in cervical dystonia. Neurology 60(7):1186–1188
R Core Team R (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria. https://www.R-project.org/. Accessed 26 Dec 2018
Hefter H, Spiess C, Rosenthal D (2014) Very early reduction in efficacy of botulinum toxin therapy for cervical dystonia in patients with subsequent secondary treatment failure: a retrospective analysis. J Neural Transm (Vienna) 121(5):513–519. https://doi.org/10.1007/s00702-013-1127-5
Jankovic J, Adler CH, Charles D, Comella C, Stacy M, Schwartz M, Manack Adams A, Brin MF (2015) Primary results from the cervical dystonia patient registry for observation of onabotulinumtoxina efficacy (CD PROBE). J Neurol Sci 349(1–2):84–93. https://doi.org/10.1016/j.jns.2014.12.030
Misra VP, Ehler E, Zakine B, Maisonobe P, Simonetta-Moreau M, group IIC (2012) Factors influencing response to Botulinum toxin type A in patients with idiopathic cervical dystonia: results from an international observational study. BMJ Open. https://doi.org/10.1136/bmjopen-2012-000881
Moll M, Rosenthal D, Hefter H (2018) Quality of life in long-term botulinum toxin treatment of cervical dystonia: results of a cross sectional study. Parkinsonism Relat Disord. https://doi.org/10.1016/j.parkreldis.2018.07.019
Misra VP, Trosch RM, Maisonobe P, Om S (2018) Spectrum of practice in the routine management of cervical dystonia with abobotulinumtoxinA: findings from three prospective open-label observational studies. J Clin Mov Disord 5:4. https://doi.org/10.1186/s40734-018-0072-8
Ramirez-Castaneda J, Jankovic J (2013) Long-term efficacy and safety of botulinum toxin injections in dystonia. Toxins (Basel) 5(2):249–266. https://doi.org/10.3390/toxins5020249
Hauser RA, Truong D, Hubble J, Coleman C, Beffy JL, Chang S, Picaut P (2013) AbobotulinumtoxinA (Dysport) dosing in cervical dystonia: an exploratory analysis of two large open-label extension studies. J Neural Transm (Vienna) 120(2):299–307. https://doi.org/10.1007/s00702-012-0872-1
Colosimo C, Tiple D, Berardelli A (2012) Efficacy and safety of long-term botulinum toxin treatment in craniocervical dystonia: a systematic review. Neurotox Res 22(4):265–273. https://doi.org/10.1007/s12640-012-9314-y
Tsui JK, Eisen A, Stoessl AJ, Calne S, Calne DB (1986) Double-blind study of botulinum toxin in spasmodic torticollis. Lancet 2(8501):245–247
Ceylan D, Erer S, Zarifoglu M, Turkes N, Ozkaya G (2019) Evaluation of anxiety and depression scales and quality of LIFE in cervical dystonia patients on botulinum toxin therapy and their relatives. Neurol Sci 40(4):725–731. https://doi.org/10.1007/s10072-019-3719-9
Albanese A, Sorbo FD, Comella C, Jinnah HA, Mink JW, Post B, Vidailhet M, Volkmann J, Warner TT, Leentjens AF, Martinez-Martin P, Stebbins GT, Goetz CG, Schrag A (2013) Dystonia rating scales: critique and recommendations. Mov Disord 28(7):874–883. https://doi.org/10.1002/mds.25579
Jost WH, Hefter H, Stenner A, Reichel G (2013) Rating scales for cervical dystonia: a critical evaluation of tools for outcome assessment of botulinum toxin therapy. J Neural Transm (Vienna) 120(3):487–496. https://doi.org/10.1007/s00702-012-0887-7
Acknowledgements
We thank Rebecca Willnecker, Sophia Schleifer and Korbinian Westphal for their support for the data capture.
Funding
There has been no funding of the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
A Jochim has received travel grants from Ipsen Pharma GmbH, Merz Pharmaceuticals GmbH, Pharm-Allergan GmbH, Boston Scientific, Bayer Health and Universitätsklinikum Würzburg as well as speaker honoraria from Pharm-Allergan GmbH. T. Meindl has received travel grants from Ipsen Pharma GmbH, Merz Pharmaceuticals GmbH, Pharm-Allergan GmbH and Boston Scientific Medizintechnik. GmbH. T. Mantel has received travel grants from Bayer Vital GmbH. S. Zwirner has no conflicts of interest. M. Zech is supported by an internal research program at Helmholtz Center Munich, Germany (“Physician Scientists for Groundbreaking Projects”), has received travel expenses from Pharm-Allergan GmbH, and has received speaker honoraria from Bayer Vital GmbH. F. Castrop has no conflicts of interest. B. Haslinger has received research support from the German Research Foundation (DFG), Pharm-Allergan GmbH and Ipsen Pharma GmbH, has received speaker honoraria from Pharm-Allergan GmbH and Bayer Health Care Pharmaceuticals and has received travel grants from Ipsen Pharma GmbH.
Ethical approval
The study has been approved by the local ethics review board. All patients whose data were not only acquired retrospectively, but also recorded prospectively, gave their written informed consent before entering the prospective phase of the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jochim, A., Meindl, T., Mantel, T. et al. Treatment of cervical dystonia with abo- and onabotulinumtoxinA: long-term safety and efficacy in daily clinical practice. J Neurol 266, 1879–1886 (2019). https://doi.org/10.1007/s00415-019-09349-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09349-2