Abstract
Background
Observational and interventional studies addressing the link between amyloid (Aβ) burden and cognitive decline are increasing, but a clear definition of amyloid positivity is still lacking. This may represent a great stake for therapeutic studies enrolling Aβ + patients only. The main objective of this study was to define a population with “equivocal” amyloid status, and evaluate their cognitive changes.
Methods
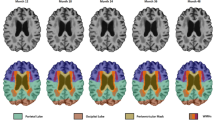
Sixty-five participants over 75 years old, from the Control group of the interventional MAPT study, at risk to develop Alzheimer’s disease, were included. Participants were classified into three groups in terms of amyloid load: Aβ +, Aβ − and Equivocal participants (according to visual reading, global standardized uptake (SUVR) cut-offs, or a k-mean clustering method). The cognitive changes over time (memory, executive functions, attention and processing speed) of this Equivocal group were then compared to Aβ + and Aβ − participants.
Results
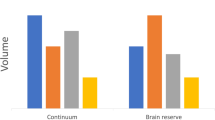
When classified by visual read, Equivocal participants’ memory scores were comparable to the Aβ- participants, and greater than in Aβ + participants over time. Secondary analyses, using SUVR cut-offs classification, showed different trajectories with Equivocal participants being comparable to the Aβ + participants, and lower than Aβ-, on executive performance over time.
Conclusions
This original work pointed out a population that may be of great interest for interventional studies, raising the question of how amyloid status should be defined and integrated in such studies. These findings should be replicated in future studies on larger datasets, to confirm what methodological approach would be the most suitable to highlight this specific neuroimaging entity.




Similar content being viewed by others
References
Herholz K, Ebmeier K (2011) Clinical amyloid imaging in Alzheimer’s disease. Lancet Neurol 10(7):667–670
Ma Y, Zhang S, Li J, Zheng DM, Guo Y, Feng J, Ren WD (2014) Predictive accuracy of amyloid imaging for progression from mild cognitive impairment to Alzheimer disease with different lengths of follow-up: a meta-analysis. Medicine 93:27
Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, … Park DC (2011) Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. J Alzheimer’s Assoc 7(3):280–292
Kemppainen NM, Aalto S, Wilson IA, Nagren K, Helin S, Bruck A et al. PET amyloid ligand [11C]PIB uptake is increased in mild cognitive impairment. Neurology. 2007;68:1603–1606. https://doi.org/10.1212/01.wnl.0000260969.94695.56
Baker JE, Lim YY, Pietrzak RH, Hassenstab J, Snyder PJ, Masters CL, Maruff P (2017) Cognitive impairment and decline in cognitively normal older adults with high amyloid-β: A meta-analysis. Alzheimer’s Dement 6:108–121
Clark CM, Schneider JA, Bedell BJ, Beach TG, Bilker WB, Mintun MA et al (2011) Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA 305:275–283. https://doi.org/10.1001/jama.2010.2008
Ng S, Villemagne VL, Berlangieri S, Lee ST, Cherk M, Gong SJ, … Smith C (2007) Visual assessment versus quantitative assessment of 11C-PIB PET and 18F-FDG PET for detection of Alzheimer’s disease. J Nucl Med 48(4):547–552
Fleisher AS, Chen K, Liu X, Roontiva A, Thiyyagura P, Ayutyanont N, Doraiswamy PM (2011) Using positron emission tomography and florbetapir F 18 to image cortical amyloid in patients with mild cognitive impairment or dementia due to Alzheimer disease. Arch Neurol 68(11):1404–1411
Landau SM, Mintun MA, Joshi AD, Koeppe RA, Petersen RC, Aisen PS, Jagust WJ (2012) Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Annal Neurol 72(4):578–586
Camus V, Payoux P, Barré L, Desgranges B, Voisin T, Tauber C, Mondon K (2012) Using PET with 18 F-AV-45 (florbetapir) to quantify brain amyloid load in a clinical environment. Eur J Nucl Med Mol Imaging 39(4):621–631
Joshi AD, Pontecorvo MJ, Clark CM, Carpenter AP, Jennings DL, Sadowsky CH, Saha K (2012) Performance characteristics of amyloid PET with florbetapir F 18 in patients with Alzheimer’s disease and cognitively normal subjects. J Nucl Med 53(3):378–384
Chiotis K, Saint-Aubert L, Savitcheva I, Jelic V, Andersen P, Eriksson J, Nordberg A (2016) Imaging in-vivo tau pathology in Alzheimer’s disease with THK5317 PET in a multimodal paradigm. Eur J Nucl Med Mol Imag 43(9):1686–1699
Mormino EC, Brandel MG, Madison CM, Rabinovici GD, Marks S, Baker SL, Jagust WJ (2012) Not quite PIB-positive, not quite PIB-negative: slight PIB elevations in elderly normal control subjects are biologically relevant. Neuroimage 59(2):1152–1160
Payoux P, Delrieu J, Gallini A, Adel D, Salabert AS, Hitzel A, Fernandez P (2015) Cognitive and functional patterns of nondemented subjects with equivocal visual amyloid PET findings. Eur J Nucl Med Mol Imag 42(9):1459–1468
Vellas B, Carrie I, Gillette-Guyonnet S, Touchon J, Dantoine T, Dartigues JF, Bories L (2014) MAPT study: a multidomain approach for preventing Alzheimer’s disease: design and baseline data. J Prevent Alzheimer’s Dis 1(1):13
Andrieu S, Guyonnet S, Coley N, Cantet C, Bonnefoy M, Bordes S, Desclaux F (2017) Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurol 16(5):377–389
Grothe MJ, Barthel H, Sepulcre J, Dyrba M, Sabri O, Teipel SJ, Jagust W (2017) In vivo staging of regional amyloid deposition. Neurology 89(20):2031–2038
Hedden T, Oh H, Younger AP, Patel TA (2013) Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology 80(14):1341–1348
Landau SM, Horng A, Jagust WJ, Alzheimer’s Disease Neuroimaging Initiative (2018) Memory decline accompanies subthreshold amyloid accumulation. Neurology 5:10–1212
Kemppainen N, Johansson J, Teuho J, Parkkola R, Joutsa J, Ngandu T, Paajanen T (2018) Brain amyloid load and its associations with cognition and vascular risk factors in FINGER Study. Neurology 90(3):e206–e213
Cohen AD, Mowrey W, Weissfeld LA, Aizenstein HJ, McDade E, Mountz JM, Williamson J (2013) Classification of amyloid-positivity in controls: comparison of visual read and quantitative approaches. Neuroimage 71:207–215
Klunk WE, Koeppe RA, Price JC, Benzinger TL, Devous MD, Jagust WJ, Rowe CC (2015) The Centiloid Project: standardizing quantitative amyloid plaque estimation by PET. Alzheimer’s Dement 11(1):1–15
Farrell ME, Kennedy KM, Rodrigue KM, Wig G, Bischof GN, Rieck JR, Park DC (2017) Association of longitudinal cognitive decline with amyloid burden in middle-aged and older adults: evidence for a dose-response relationship. JAMA Neurol 74(7):830–838
Chételat G, Villemagne VL, Villain N, Jones G, Ellis KA, Ames D, Rowe CC (2012) Accelerated cortical atrophy in cognitively normal elderly with high β-amyloid deposition. Neurology 78(7):477–484
McMillan CT, Chételat G. Amyloid “accumulators”: the next generation of candidates for amyloid-targeted clinical trials? [published online March 23, 2018]. Neurology. https://doi.org/10.1212/WNL.0000000000005362
Acknowledgements
This study was supported by grants from the French Ministry of Health (PHRC 2008), and the Institut de Recherche Pierre Fabre. The promotion of this study was supported by the University Hospital Center of Toulouse. Biological sample collection was supported by Exhonit Therapeutics. The AV45-MAPT study was supported by Avid radiopharmaceuticals/Eli Lilly and Company. Authors would like to thank all the members of the MAPT/DSA Study Group.
Funding
This work was funded by grants from the Gérontopôle of Toulouse, the French Ministry of Health (PHRC 2008, 2009), Pierre Fabre Research Institute, Exhonit Therapeutics SA, and Avid Radiopharmaceuticals Inc.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
Prof. P. Payoux served on the scientific advisory board of Avid Radiopharmaceuticals and GEHC. Dr. J. Delrieu served on the scientific advisory board of Avid Radiopharmaceuticals. Prof. B. Vellas served on the scientific advisory board of Avid Radiopharmaceuticals. Other authors (Dr K. Pothier, Dr L. Saint-Aubert, Dr C. Hooper, Dr de Souto Baretto) declare that they have no conflict of interest.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pothier, K., Saint-Aubert, L., Hooper, C. et al. Cognitive changes of older adults with an equivocal amyloid load. J Neurol 266, 835–843 (2019). https://doi.org/10.1007/s00415-019-09203-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09203-5