Abstract
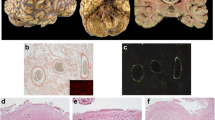
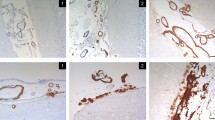
Familial amyloid polyneuropathies (FAPs) are life-threatening, autosomal dominant diseases resulting, in most instances, from transthyretin gene (TTR) variants. A small number of TTR variants lead to leptomeningeal amyloidosis (LA), which is a rare FAP subtype with late-onset central nervous system (CNS) impairment symptoms. Previous studies suggest that LA’s CNS selectivity was due to complete endoplasmic reticulum-associated degradation of highly destabilized mutants in peripheral tissues. LA’s later age at onset (AAO) was due to lower choroid plexus secretory efficacy. This study reports on a family with LA, including six symptomatic and three presymptomatic members. The LA diagnosis was confirmed by leptomeningeal enhancement on contrast MRI, elevated cerebrospinal fluid protein levels, and positive Congo red staining. The predominant symptoms included headaches, dizziness, vomiting, hallucinations, and cognitive impairments which associated with obstructive hydrocephalus. The TTR p.D38G variant with the lowest secretory efficacy was identified as the genetic cause by whole exome sequencing. The family had a statistically significantly earlier mean AAO of 31.3 ± 7.4 (p = 0.001). These uncommon phenotypes indicate unknown factors influencing the progress of CNS impairment via TTR mutants. Medical imaging examinations suggest the potential early diagnosis value of contrast MRI and the importance of ependyma involvement in LA. LA genetic and clinical data were reviewed and summarized. These findings expand the FAPs’ phenotypic spectrum and are valuable in FAP diagnosis, treatment, and further research.



Similar content being viewed by others
References
Planté-Bordeneuve V, Said G (2011) Familial amyloid polyneuropathy. Lancet Neurol 10(12):1086–1097. https://doi.org/10.1016/S1474-4422(11)70246-0
Sekijima Y (2015) Transthyretin (ATTR) amyloidosis: clinical spectrum, molecular pathogenesis and disease-modifying treatments. J Neurol Neurosurg Psychiatry 86(9):1036–1043. https://doi.org/10.1136/jnnp-2014-308724
Hund E, Linke RP, Willig F, Grau A (2001) Transthyretin-associated neuropathic amyloidosis. Pathogenesis and treatment. Neurology 56(4):431–435. https://doi.org/10.1212/WNL.56.4.431
Chen Q, Yuan L, Deng X, Yang Z, Zhang S, Deng S, Lu H, Deng H (2018) A missense variant p.Ala117Ser in the transthyretin gene of a Han Chinese family with familial amyloid polyneuropathy. Mol Neurobiol 55(6):4911–4917. https://doi.org/10.1007/s12035-017-0694-0
Sekijima Y, Yoshida K, Tokuda T, Ikeda S (1993) Familial transthyretin amyloidosis. In: Adam MP, Ardinger HH, Pagon RA et al (eds) GeneReviews. Seattle (WA). http://www.ncbi.nlm.nih.gov/pubmed/20301373
Eisele YS, Monteiro C, Fearns C, Encalada SE, Wiseman RL, Powers ET, Kelly JW (2015) Targeting protein aggregation for the treatment of degenerative diseases. Nat Rev Drug Discov 14(11):759–780. https://doi.org/10.1038/nrd4593
Tawara S, Nakazato M, Kangawa K, Matsuo H, Araki S (1983) Identification of amyloid prealbumin variant in familial amyloidotic polyneuropathy (Japanese type). Biochem Biophys Res Commun 116(3):880–888. https://doi.org/10.1016/S0006-291X(83)80224-1
Ando Y, Coelho T, Berk JL, Cruz MW, Ericzon BG, Ikeda S, Lewis WD, Obici L et al (2013) Guideline of transthyretin-related hereditary amyloidosis for clinicians. Orphanet J Rare Dis 8:31. https://doi.org/10.1186/1750-1172-8-31
Parman Y, Adams D, Obici L, Galán L, Guergueltcheva V, Suhr OB, Coelho T (2016) Sixty years of transthyretin familial amyloid polyneuropathy (TTR-FAP) in Europe: where are we now? A European network approach to defining the epidemiology and management patterns for TTR-FAP. Curr Opin Neurol 29(Suppl 1):S3–S13. https://doi.org/10.1097/WCO.0000000000000288
Garzuly F, Vidal R, Wisniewski T, Brittig F, Budka H (1996) Familial meningocerebrovascular amyloidosis, Hungarian type, with mutant transthyretin (TTR Asp18Gly). Neurology 47(6):1562–1567. https://doi.org/10.1212/WNL.47.6.1562
Maia LF, Magalhães R, Freitas J, Taipa R, Pires MM, Osório H, Dias D, Pessegueiro H et al (2015) CNS involvement in V30M transthyretin amyloidosis: clinical, neuropathological and biochemical findings. J Neurol Neurosurg Psychiatry 86(2):159–167. https://doi.org/10.1136/jnnp-2014-308107
Nakamura M, Yamashita T, Ueda M, Obayashi K, Sato T, Ikeda T, Washimi Y, Hirai T et al (2005) Neuroradiologic and clinicopathologic features of oculoleptomeningeal type amyloidosis. Neurology 65(7):1051–1056. https://doi.org/10.1212/01.wnl.0000178983.20975.af
Yuan L, Deng X, Song Z, Yang Z, Ni B, Chen Y, Deng H (2015) Genetic analysis of the RAB39B gene in Chinese Han patients with Parkinson’s disease. Neurobiol Aging 36(10):2907.e11–2907.e12. https://doi.org/10.1016/j.neurobiolaging.2015.06.019
Mitsuhashi S, Yazaki M, Tokuda T, Sekijima Y, Washimi Y, Shimizu Y, Ando Y, Benson MD et al (2005) Biochemical characteristics of variant transthyretins causing hereditary leptomeningeal amyloidosis. Amyloid 12(4):216–225. https://doi.org/10.1080/13506120500352404
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17(5):405–424. https://doi.org/10.1038/gim.2015.30
Sekijima Y, Wiseman RL, Matteson J, Hammarström P, Miller SR, Sawkar AR, Balch WE, Kelly JW (2005) The biological and chemical basis for tissue-selective amyloid disease. Cell 121(1):73–85. https://doi.org/10.1016/j.cell.2005.01.018
Jin K, Sato S, Takahashi T, Nakazaki H, Date Y, Nakazato M, Tominaga T, Itoyama Y et al (2004) Familial leptomeningeal amyloidosis with a transthyretin variant Asp18Gly representing repeated subarachnoid haemorrhages with superficial siderosis. J Neurol Neurosurg Psychiatry 75(10):1463–1466. https://doi.org/10.1136/jnnp.2003.029942
Bevers MB, McGuone D, Jerath NU, Musolino PL (2016) Leptomeningeal transthyretin-type amyloidosis presenting as acute hydrocephalus and subarachnoid hemorrhage. J Clin Neurosci 29:203–205. https://doi.org/10.1016/j.jocn.2015.12.017
Brett M, Persey MR, Reilly MM, Revesz T, Booth DR, Booth SE, Hawkins PN, Pepys MB et al (1999) Transthyretin Leu12Pro is associated with systemic, neuropathic and leptomeningeal amyloidosis. Brain 122(Pt2):183–190. https://doi.org/10.1093/brain/122.2.183
Barreiros AP, Post F, Hoppe-Lotichius M, Linke RP, Vahl CF, Schäfers HJ, Galle PR, Otto G (2010) Liver transplantation and combined liver-heart transplantation in patients with familial amyloid polyneuropathy: a single-center experience. Liver Transpl 16(3):314–323. https://doi.org/10.1002/lt.21996
McColgan P, Viegas S, Gandhi S, Bull K, Tudor R, Sheikh F, Pinney J, Fontana M et al (2015) Oculoleptomeningeal amyloidosis associated with transthyretin Leu12Pro in an African patient. J Neurol 262(1):228–234. https://doi.org/10.1007/s00415-014-7594-2
Shimizu Y, Takeuchi M, Matsumura M, Tokuda T, Iwata M (2006) A case of biopsy-proven leptomeningeal amyloidosis and intravenous Ig-responsive polyneuropathy associated with the Ala25Thr transthyretin gene mutation. Amyloid 13(1):37–41. https://doi.org/10.1080/13506120600551814
Hagiwara K, Ochi H, Suzuki S, Shimizu Y, Tokuda T, Murai H, Shigeto H, Ohyagi Y et al (2009) Highly selective leptomeningeal amyloidosis with transthyretin variant Ala25Thr. Neurology 72(15):1358–1360. https://doi.org/10.1212/WNL.0b013e3181a0fe74
Llull L, Berenguer J, Yagüe J, Graus F (2014) Leptomeningeal amyloidosis due to A25T TTR mutation: a case report. Neurologia 29(6):382–384. https://doi.org/10.1016/j.nrl.2012.12.006
Herrick MK, DeBruyne K, Horoupian DS, Skare J, Vanefsky MA, Ong T (1996) Massive leptomeningeal amyloidosis associated with a Val30Met transthyretin gene. Neurology 47(4):988–992. https://doi.org/10.1212/WNL.47.4.988
Dowell JD, Fleck JD, Vakili ST, Benson MD (2007) Familial oculoleptomeningeal amyloidosis associated with primary angiitis of the CNS. Neurology 68(1):77–78. https://doi.org/10.1212/01.wnl.0000250343.34110.79
Goren H, Steinberg MC, Farboody GH (1980) Familial oculoleptomeningeal amyloidosis. Brain 103(3):473–495. https://doi.org/10.1093/brain/103.3.473
Petersen RB, Goren H, Cohen M, Richardson SL, Tresser N, Lynn A, Gali M, Estes M et al (1997) Transthyretin amyloidosis: a new mutation associated with dementia. Ann Neurol 41(3):307–313. https://doi.org/10.1002/ana.410410305
Roe RH, Fisher Y, Eagle RC, Fine HF, Cunningham ET (2007) Oculoleptomeningeal amyloidosis in a patient with a TTR Val30Gly mutation in the transthyretin gene. Ophthalmology 114(11):e33–e37. https://doi.org/10.1016/j.ophtha.2007.07.007
Liu JK, Turner RD, Luciano MG, Krishnaney AA (2015) Circumferential intrathecal ossification in oculoleptomeningeal amyloidosis. J Clin Neurosci 22(4):769–771. https://doi.org/10.1016/j.jocn.2014.10.021
Martin SE, Benson MD, Hattab EM (2014) The pathologic spectrum of oculoleptomeningeal amyloidosis with Val30Gly transthyretin gene mutation in a postmortem case. Hum Pathol 45(5):1105–1108. https://doi.org/10.1016/j.humpath.2013.10.037
Mascalchi M, Salvi F, Pirini MG, D’Errico A, Ferlini A, Lolli F, Plasmati R, Tessa C et al (1999) Transthyretin amyloidosis and superficial siderosis of the CNS. Neurology 53(7):1498–1503. https://doi.org/10.1212/WNL.53.7.1498
Klein CJ, Nakumura M, Jacobson DR, Lacy MQ, Benson MD, Petersen RC (1998) Transthyretin amyloidosis (serine 44) with headache, hearing loss, and peripheral neuropathy. Neurology 51(5):1462–1464. https://doi.org/10.1212/WNL.51.5.1462
Nakagawa K, Sheikh SI, Snuderl M, Frosch MP, Greenberg SM (2008) A new Thr49Pro transthyretin gene mutation associated with leptomeningeal amyloidosis. J Neurol Sci 272(1–2):186–190. https://doi.org/10.1016/j.jns.2008.05.014
Ellie E, Camou F, Vital A, Rummens C, Grateau G, Delpech M, Valleix S (2001) Recurrent subarachnoid hemorrhage associated with a new transthyretin variant (Gly53Glu). Neurology 57(1):135–137. https://doi.org/10.1212/WNL.57.1.135
Douglass C, Suvarna K, Reilly MM, Hawkins PN, Hadjivassiliou M (2007) A novel amyloidogenic transthyretin variant, Gly53Ala, associated with intermittent headaches and ataxia. J Neurol Neurosurg Psychiatry 78(2):193–195. https://doi.org/10.1136/jnnp.2006.093500
Liepnieks JJ, Dickson DW, Benson MD (2011) A new transthyretin mutation associated with leptomeningeal amyloidosis. Amyloid 18(Suppl 1):160–162. https://doi.org/10.3109/13506129.2011.574354060
Uitti RJ, Donat JR, Rozdilsky B, Schneider RJ, Koeppen AH (1988) Familial oculoleptomeningeal amyloidosis. Report of a new family with unusual features. Arch Neurol 45(10):1118–1122. https://doi.org/10.1001/archneur.1988.00520340072015
Uemichi T, Uitti RJ, Koeppen AH, Donat JR, Benson MD (1999) Oculoleptomeningeal amyloidosis associated with a new transthyretin variant Ser64. Arch Neurol 56(9):1152–1155. https://doi.org/10.1001/archneur.56.9.1152
Blevins G, Macaulay R, Harder S, Fladeland D, Yamashita T, Yazaki M, Hamidi Asl K, Benson MD et al (2003) Oculoleptomeningeal amyloidosis in a large kindred with a new transthyretin variant Tyr69His. Neurology 60(10):1625–1630. https://doi.org/10.1212/01.WNL.0000065901.18353.AB
Schweitzer K, Ehmann D, Garcia R, Alport E (2009) Oculoleptomeningeal amyloidosis in 3 individuals with the transthyretin variant Tyr69His. Can J Ophthalmol 44(3):317–319. https://doi.org/10.3129/i09-023
Suhr OB, Andersen O, Aronsson T, Jonasson J, Kalimo H, Lundahl C, Lundgren HE, Melberg A et al (2009) Report of five rare or previously unknown amyloidogenic transthyretin mutations disclosed in Sweden. Amyloid 16(4):208–214. https://doi.org/10.3109/13506120903421587
Ziskin JL, Greicius MD, Zhu W, Okumu AN, Adams CM, Plowey ED (2015) Neuropathologic analysis of Tyr69His TTR variant meningovascular amyloidosis with dementia. Acta Neuropathol Commun 3:43. https://doi.org/10.1186/s40478-015-0216-0
Zeldenrust SR, Skinner M, Share J, Benson MD (1994) A new transthyretin variant (His 69) associated with vitreous amyloid in an FAP family. Amyloid 1(1):17–22. https://doi.org/10.3109/13506129409148620
Purrucker JC, Hund E, Hinderhofer K, Kollmer J, Schönland S, Hegenbart U (2013) Doxycycline in ATTRY69H (p.ATTRY89H) amyloidosis with predominant leptomeningeal manifestation. Amyloid 20(4):279–280. https://doi.org/10.3109/13506129.2013.829439
Mathieu F, Morgan E, So J, Munoz DG, Mason W, Kongkham P (2018) Oculoleptomeningeal amyloidosis secondary to the rare transthyretin c.381T> G (p.Ile127Met) mutation. World Neurosurg 111:190–193. https://doi.org/10.1016/j.wneu.2017.12.096
Connors LH, Lim A, Prokaeva T, Roskens VA, Costello CE (2003) Tabulation of human transthyretin (TTR) variants, 2003. Amyloid 10(3):160–184. https://doi.org/10.3109/13506120308998998
Benson MD, Kincaid JC (2007) The molecular biology and clinical features of amyloid neuropathy. Muscle Nerve 36(4):411–423. https://doi.org/10.1002/mus.20821
Salvi F, Pastorelli F, Plasmati R, Bartolomei I, Dall’Osso D, Rapezzi C (2012) Genotypic and phenotypic correlation in an Italian population of hereditary amyloidosis TTR-related (HA-TTR): clinical and neurophysiological aids to diagnosis and some reflections on misdiagnosis. Amyloid 19(Suppl 1):58–60. https://doi.org/10.3109/13506129.2012.682187
Stabile A, Di Lazzaro V, Colosimo C, Piazza F, Ferrarese C, Di Francesco JC (2018) Idiopathic infratentorial superficial siderosis of the central nervous system: case report and review of literature. Neurol Neurochir Pol 52(1):102–106. https://doi.org/10.1016/j.pjnns.2017.10.006
Funding
H.D.’s research was supported by grants from National Key Research and Development Program of China (2016YFC1306604), National Natural Science Foundation of China (81670216 and 81873686), Natural Science Foundation of Hunan Province (2016JJ2166), Scientific Research Project of Health and Family Planning Commission of Hunan Province (B20180760), Grant for the Foster Key Subject of the Third Xiangya Hospital of Central South University (Clinical Laboratory Diagnostics), the New Xiangya Talent Project of the Third Xiangya Hospital of Central South University (20150301), China. L.Y.’s research was supported by grants from National Natural Science Foundation of China (81800219), Scientific Research Project of Health and Family Planning Commission of Hunan Province (B20180729), China.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical standards
The study received approval from the Ethics Committee of the Third Xiangya Hospital and was performed in accordance with the ethical standards stated in the 1964 Declaration of Helsinki.
Informed consent
Informed consent was obtained from the individuals.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fan, K., Zhu, H., Xu, H. et al. The identification of a transthyretin variant p.D38G in a Chinese family with early-onset leptomeningeal amyloidosis. J Neurol 266, 232–241 (2019). https://doi.org/10.1007/s00415-018-9125-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-9125-z