Abstract
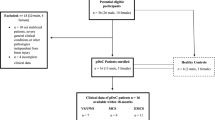
Previous studies could demonstrate that functional magnetic resonance imaging (fMRI), fludeoxyglucose positron emission tomography (FDG-PET), and electroencephalography (EEG) measures contain information about patients suffering from disorders of consciousness (DOC) and thus improve the clinical diagnosis. Additionally, the technical modalities were able to predict the outcome of patients. However, most studies lack proven reproducibility in a clinical setting. We here applied a standardized combined EEG/fMRI/FDG-PET measurement to a cohort of 20 patients suffering from DOC and focused on parameters that have been demonstrated to contain information about diagnosis and prognosis of these patients. We evaluated EEG band power, fMRI connectivity in networks associated with consciousness and sensory networks, as well as absolute glucose uptake in the brain as potential markers of preserved consciousness or favorable outcome. Acquired data were analyzed by a principal component analysis to identify the most important markers in a hypothesis-free manner. These were then analyzed with statistical group comparisons. Absolute FDG-PET could prove that glucose metabolism in the occipital lobe is significantly higher in minimally conscious than in vegetative state patients. Delta band power showed to be prognostic marker for a favorable outcome. We conclude that absolute FDG-PET is a suitable tool to evaluate the level consciousness in DOC patients. Additionally, we propose delta band power as marker of a favorable outcome in DOC patients. We suggest that these findings promote a standardized technical evaluation of DOC patients to improve diagnosis and prognosis.




Similar content being viewed by others
References
Alkire MT, Haier RJ, Barker SJ, Shah NK, Wu JC, Kao YJ (1995) Cerebral metabolism during propofol anesthesia in humans studied with positron emission tomography. Anesthesiology 82:393–403 (discussion 327A)
Alkire MT, Haier RJ, Shah NK, Anderson CT (1997) Positron emission tomography study of regional cerebral metabolism in humans during isoflurane anesthesia. Anesthesiology 86:549–557
Bagnato S, Boccagni C, Sant’Angelo A, Prestandrea C, Mazzilli R, Galardi G (2015) EEG predictors of outcome in patients with disorders of consciousness admitted for intensive rehabilitation. Clin Neurophysiol 126:959–966
Bodart O, Laureys S (2014) Predicting outcome from subacute unresponsive wakefulness syndrome or vegetative state. Crit Care 18:132
Boveroux P, Vanhaudenhuyse A, Bruno MA, Noirhomme Q, Lauwick S, Luxen A, Degueldre C, Plenevaux A, Schnakers C, Phillips C, Brichant JF, Bonhomme V, Maquet P, Greicius MD, Laureys S, Boly M (2010) Breakdown of within- and between-network resting state functional magnetic resonance imaging connectivity during propofol-induced loss of consciousness. Anesthesiology 113:1038–1053
Casarotto S, Comanducci A, Rosanova M, Sarasso S, Fecchio M, Napolitani M, Pigorini A, GC A, Trimarchi PD, Boly M, Gosseries O, Bodart O, Curto F, Landi C, Mariotti M, Devalle G, Laureys S, Tononi G, Massimini M (2016) Stratification of unresponsive patients by an independently validated index of brain complexity. Ann Neurol 80:718–729
Chao-Gan Y, Yu-Feng Z (2010) DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-State fMRI. Front Syst Neurosci 4:13
Cruse D, Chennu S, Fernandez-Espejo D, Payne WL, Young GB, Owen AM (2012) Detecting awareness in the vegetative state: electroencephalographic evidence for attempted movements to command. PLoS One 7:e49933
Demertzi A, Antonopoulos G, Heine L, Voss HU, Crone JS, de Los Angeles C, Bahri MA, Di Perri C, Vanhaudenhuyse A, Charland-Verville V, Kronbichler M, Trinka E, Phillips C, Gomez F, Tshibanda L, Soddu A, Schiff ND, Whitfield-Gabrieli S, Laureys S (2015) Intrinsic functional connectivity differentiates minimally conscious from unresponsive patients. Brain 138:2619–2631
Demertzi A, Gomez F, Crone JS, Vanhaudenhuyse A, Tshibanda L, Noirhomme Q, Thonnard M, Charland-Verville V, Kirsch M, Laureys S, Soddu A (2014) Multiple fMRI system-level baseline connectivity is disrupted in patients with consciousness alterations. Cortex 52:35–46
DeVolder AG, Goffinet AM, Bol A, Michel C, de Barsy T, Laterre C (1990) Brain glucose metabolism in postanoxic syndrome. Positron emission tomographic study. Arch Neurol 47:197–204
Edgren E, Enblad P, Grenvik A, Lilja A, Valind S, Wiklund L, Hedstrand U, Stjernstrom H, Persson L, Ponten U, Langstrom B (2003) Cerebral blood flow and metabolism after cardiopulmonary resuscitation. A pathophysiologic and prognostic positron emission tomography pilot study. Resuscitation 57:161–170
Estraneo A, Moretta P, Loreto V, Lanzillo B, Cozzolino A, Saltalamacchia A, Lullo F, Santoro L, Trojano L (2013) Predictors of recovery of responsiveness in prolonged anoxic vegetative state. Neurology 80:464–470
Fingelkurts AA, Fingelkurts AA, Bagnato S, Boccagni C, Galardi G (2013) Prognostic value of resting-state electroencephalography structure in disentangling vegetative and minimally conscious states: a preliminary study. Neurorehabil Neural Repair 27:345–354
Fischer C, Luaute J, Nemoz C, Morlet D, Kirkorian G, Mauguiere F (2006) Improved prediction of awakening or nonawakening from severe anoxic coma using tree-based classification analysis. Crit Care Med 34:1520–1524
Giacino JT, Schnakers C, Rodriguez-Moreno D, Kalmar K, Schiff N, Hirsch J (2009) Behavioral assessment in patients with disorders of consciousness: gold standard or fool’s gold? Prog Brain Res 177:33–48
Gosseries O, Schnakers C, Ledoux D, Vanhaudenhuyse A, Bruno MA, Demertzi A, Noirhomme Q, Lehembre R, Damas P, Goldman S, Peeters E, Moonen G, Laureys S (2011) Automated EEG entropy measurements in coma, vegetative state/unresponsive wakefulness syndrome and minimally conscious state. Funct Neurol 26:25–30
Graham MM, Muzi M, Spence AM, O’Sullivan F, Lewellen TK, Link JM, Krohn KA (2002) The FDG lumped constant in normal human brain. J Nucl Med 43:1157–1166
Holler Y, Thomschewski A, Bergmann J, Kronbichler M, Crone JS, Schmid EV, Butz K, Holler P, Nardone R, Trinka E (2014) Connectivity biomarkers can differentiate patients with different levels of consciousness. Clin Neurophysiol 125:1545–1555
Howell K, Grill E, Klein AM, Straube A, Bender A (2013) Rehabilitation outcome of anoxic-ischaemic encephalopathy survivors with prolonged disorders of consciousness. Resuscitation 84:1409–1415
Izquierdo-Garcia D, Catana C (2016) MR imaging-guided attenuation correction of PET data in PET/MR imaging. PET Clin 11:129–149
Jordan D, Ilg R, Riedl V, Schorer A, Grimberg S, Neufang S, Omerovic A, Berger S, Untergehrer G, Preibisch C, Schulz E, Schuster T, Schroter M, Spoormaker V, Zimmer C, Hemmer B, Wohlschlager A, Kochs EF, Schneider G (2013) Simultaneous electroencephalographic and functional magnetic resonance imaging indicate impaired cortical top–down processing in association with anesthetic-induced unconsciousness. Anesthesiology 119:1031–1042
Kaisti KK, Langsjo JW, Aalto S, Oikonen V, Sipila H, Teras M, Hinkka S, Metsahonkala L, Scheinin H (2003) Effects of sevoflurane, propofol, and adjunct nitrous oxide on regional cerebral blood flow, oxygen consumption, and blood volume in humans. Anesthesiology 99:603–613
Kalmar K, Giacino JT (2005) The JFK coma recovery scale-revised. Neuropsychol Rehabil 15:454–460
Khaburzania M, Beridze M (2013) Prognostic value of EEG in different etiological types of coma. Georgian Med News (219):40–46
Levy DE, Sidtis JJ, Rottenberg DA, Jarden JO, Strother SC, Dhawan V, Ginos JZ, Tramo MJ, Evans AC, Plum F (1987) Differences in cerebral blood flow and glucose utilization in vegetative versus locked-in patients. Ann Neurol 22:673–682
Monti MM, Vanhaudenhuyse A, Coleman MR, Boly M, Pickard JD, Tshibanda L, Owen AM, Laureys S (2010) Willful modulation of brain activity in disorders of consciousness. N Engl J Med 362:579–589
Muschelli J, Nebel MB, Caffo BS, Barber AD, Pekar JJ, Mostofsky SH (2014) Reduction of motion-related artifacts in resting state fMRI using aCompCor. Neuroimage 96:22–35
Owen AM, Coleman MR, Boly M, Davis MH, Laureys S, Pickard JD (2006) Detecting awareness in the vegetative state. Science 313:1402
Patlak CS, Blasberg RG (1985) Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metab 5:584–590
Ranft A, Golkowski D, Kiel T, Riedl V, Kohl P, Rohrer G, Pientka J, Berger S, Thul A, Maurer M, Preibisch C, Zimmer C, Mashour GA, Kochs EF, Jordan D, Ilg R (2016) Neural correlates of sevoflurane-induced unconsciousness identified by simultaneous functional magnetic resonance imaging and electroencephalography. Anesthesiology 125:861–872
Riedl V, Bienkowska K, Strobel C, Tahmasian M, Grimmer T, Forster S, Friston KJ, Sorg C, Drzezga A (2014) Local activity determines functional connectivity in the resting human brain: a simultaneous FDG-PET/fMRI study. J Neurosci 34:6260–6266
Rossi Sebastiano D, Panzica F, Visani E, Rotondi F, Scaioli V, Leonardi M, Sattin D, D’Incerti L, Parati E, Ferini Strambi L, Franceschetti S (2015) Significance of multiple neurophysiological measures in patients with chronic disorders of consciousness. Clin Neurophysiol 126:558–564
Rudolf J, Ghaemi M, Ghaemi M, Haupt WF, Szelies B, Heiss WD (1999) Cerebral glucose metabolism in acute and persistent vegetative state. J Neurosurg Anesthesiol 11:17–24
Santin G, Strul D, Lazaro D, Simon L, Krieguer M, Martins MV, Breton V, Morel C (2002) GATE, a Geant4-based simulation platform for PET integrating movement and time management. In: Nuclear science symposium conference record, 2002 IEEE. IEEE, pp 1325–1329
Schnakers C, Vanhaudenhuyse A, Giacino J, Ventura M, Boly M, Majerus S, Moonen G, Laureys S (2009) Diagnostic accuracy of the vegetative and minimally conscious state: clinical consensus versus standardized neurobehavioral assessment. BMC Neurol 9:35
Schorr B, Schlee W, Arndt M, Bender A (2016) Coherence in resting-state EEG as a predictor for the recovery from unresponsive wakefulness syndrome. J Neurol 263:937–953
Silva S, de Pasquale F, Vuillaume C, Riu B, Loubinoux I, Geeraerts T, Seguin T, Bounes V, Fourcade O, Demonet JF, Peran P (2015) Disruption of posteromedial large-scale neural communication predicts recovery from coma. Neurology 85:2036–2044
Sitt JD, King JR, El Karoui I, Rohaut B, Faugeras F, Gramfort A, Cohen L, Sigman M, Dehaene S, Naccache L (2014) Large scale screening of neural signatures of consciousness in patients in a vegetative or minimally conscious state. Brain 137:2258–2270
Stender J, Gosseries O, Bruno MA, Charland-Verville V, Vanhaudenhuyse A, Demertzi A, Chatelle C, Thonnard M, Thibaut A, Heine L, Soddu A, Boly M, Schnakers C, Gjedde A, Laureys S (2014) Diagnostic precision of PET imaging and functional MRI in disorders of consciousness: a clinical validation study. Lancet 384:514–522
Stender J, Kupers R, Rodell A, Thibaut A, Chatelle C, Bruno MA, Gejl M, Bernard C, Hustinx R, Laureys S, Gjedde A (2015) Quantitative rates of brain glucose metabolism distinguish minimally conscious from vegetative state patients. J Cereb Blood Flow Metab 35:58–65
Stender J, Mortensen KN, Thibaut A, Darkner S, Laureys S, Gjedde A, Kupers R (2016) The minimal energetic requirement of sustained awareness after brain injury. Curr Biol 26:1494–1499
Steppacher I, Eickhoff S, Jordanov T, Kaps M, Witzke W, Kissler J (2013) N400 predicts recovery from disorders of consciousness. Ann Neurol 73:594–602
Thul A, Lechinger J, Donis J, Michitsch G, Pichler G, Kochs EF, Jordan D, Ilg R, Schabus M (2016) EEG entropy measures indicate decrease of cortical information processing in disorders of consciousness. Clin Neurophysiol 127:1419–1427
Tommasino C, Grana C, Lucignani G, Torri G, Fazio F (1995) Regional cerebral metabolism of glucose in comatose and vegetative state patients. J Neurosurg Anesthesiol 7:109–116
Tzovara A, Rossetti AO, Spierer L, Grivel J, Murray MM, Oddo M, De Lucia M (2013) Progression of auditory discrimination based on neural decoding predicts awakening from coma. Brain 136:81–89
Vanhaudenhuyse A, Noirhomme Q, Tshibanda LJ, Bruno MA, Boveroux P, Schnakers C, Soddu A, Perlbarg V, Ledoux D, Brichant JF, Moonen G, Maquet P, Greicius MD, Laureys S, Boly M (2010) Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain 133:161–171
Villien M, Wey HY, Mandeville JB, Catana C, Polimeni JR, Sander CY, Zurcher NR, Chonde DB, Fowler JS, Rosen BR, Hooker JM (2014) Dynamic functional imaging of brain glucose utilization using fPET-FDG. Neuroimage 100:192–199
Wang F, Di H, Hu X, Jing S, Thibaut A, Di Perri C, Huang W, Nie Y, Schnakers C, Laureys S (2015) Cerebral response to subject’s own name showed high prognostic value in traumatic vegetative state. BMC Med 13:83
Wang XJ (2010) Neurophysiological and computational principles of cortical rhythms in cognition. Physiol Rev 90:1195–1268
Whitfield-Gabrieli S, Nieto-Castanon A (2012) Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2:125–141
Wu X, Zou Q, Hu J, Tang W, Mao Y, Gao L, Zhu J, Jin Y, Wu X, Lu L, Zhang Y, Zhang Y, Dai Z, Gao JH, Weng X, Zhou L, Northoff G, Giacino JT, He Y, Yang Y (2015) Intrinsic functional connectivity patterns predict consciousness level and recovery outcome in acquired brain injury. J Neurosci 35:12932–12946
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical standards
The study protocol was approved by the ethical committee of the Klinikum rechts der Isar and the study was conducted according to the Declaration of Helsinki. Every patient provided written informed consent before entering the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
415_2017_8591_MOESM1_ESM.pdf
Supplementary Table 1 Table showing the demographic and clinical data of the study cohort. Abbreviations are: sex f = female, m = male; etiology traumatic brain injury = 1, stroke = 2, anoxia = 3, metabolic/infectious cause = 4. Total CRS-R is on the date of PET/MR measurement (PDF 38 kb)
Rights and permissions
About this article
Cite this article
Golkowski, D., Merz, K., Mlynarcik, C. et al. Simultaneous EEG–PET–fMRI measurements in disorders of consciousness: an exploratory study on diagnosis and prognosis. J Neurol 264, 1986–1995 (2017). https://doi.org/10.1007/s00415-017-8591-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-017-8591-z