Abstract
Seizures after intracerebral hemorrhage are repeatedly seen. Whether the development of seizures after intracerebral hemorrhage affects survival in the long term is unknown. This study aims to determine the relation between seizures (i.e., with and without anti-epileptic therapy) and long-term mortality risk in a large patient population with intracerebral hemorrhage. We retrospectively included patients with a non-traumatic ICH in all three hospitals in the South Limburg region in the Netherlands between January 1st 2004 and December 31st 2009, and we assessed all-cause mortality until March 14th 2016. Patient who did not survive the first seven days after intracerebral hemorrhage were excluded from analyses. We used Cox multivariate analyses to determine independent predictors of mortality. Of 1214 patients, 783 hemorrhagic stroke patients fulfilled the inclusion criteria, amongst whom 37 (4.7%) patients developed early seizures (within 7 days after hemorrhage) and 77 (9.8%) developed late seizures (more than 7 days after hemorrhage). Seizure development was not significantly related to mortality risk after correction for conventional vascular risk factors and hemorrhage severity. However, we found a small but independent relation between the use of anti-epileptic drugs and a lower long-term mortality (HR = 0.32, 95% CI 0.11–0.91). In our large population, seizures and epilepsy did not relate independently to an increased mortality risk after hemorrhage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intracerebral hemorrhage (ICH) accounts for roughly 10–20% of all strokes, and has a 10-year survival of only 24% [1,2,3,4]. Furthermore, survivors of an ICH have a 10% risk to develop seizures [5, 6]. These seizures may develop within seven days (i.e., early, acute symptomatic seizures), or later (i.e., late, remote symptomatic seizures) [7]. The development of seizures may inflict additional cerebral damage, and hence increase mortality risks after ICH. However, seizure development might also coincide with important risk factors for mortality (e.g., old age, stroke severity, cortical involvement, and alcohol consumption) [6, 8, 9].
Previous studies showed conflicting findings with regard to the relation between early and late seizures, and mortality, mostly based on differences in follow-up, definition of seizures, number of included patients, and inhomogeneous patient populations [6, 10, 12,13,14,15,16,17,18]. Furthermore, early, late, and recurrent seizures differ in pathogenesis and might also have a different relation with mortality [10, 11]. Because of the inconclusive evidence, we aimed at defining the long-term mortality risk after ICH in a large population, and relate this to post-stroke seizures. We hypothesized that the development of seizures negatively influences the survival of patients after ICH.
Methods
The present study is conducted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE statement) [19].
Participants and study design
We conducted a retrospective cohort study in all three hospitals in the South Limburg region of the Netherlands (i.e., hospitals of Maastricht, Sittard, and Heerlen). In agreement with the Dutch legislation, approval of the medical ethical committees was not necessary. We included all patients with a non-traumatic ICH, older than 18 years, between January 1st 2004 and December 31st 2009. All intracerebral hemorrhages were confirmed with at least CT-imaging [20]. We excluded secondary ICH and hemorrhagic transformation of intracerebral infarcts, non-parenchymatous hemorrhage (e.g., subdural, epidural, subarachnoid, and primary intraventricular hemorrhage), or hemorrhage associated with a brain tumor or encephalitis. We also excluded patients who did not survive the first seven days to prevent information bias, as these patients are not at risk of suffering from late seizures. Furthermore, we excluded patients with pre-existing epilepsy and concurrent use of anti-epileptic drugs, as assessed from records from previous out-patient department visits, to focus on hemorrhage-associated seizures.
Variables, data sources and measurements
From hospital electronic patient records, especially looking for entries by neurologists, we retraced the occurrence of possible seizures both during hospitalization and during post-hospitalization out-patient visits and emergency department contacts in the same three hospitals by extracting reports from the treating neurologists. These possible seizures were assessed by two independent neurologists specialized in epilepsy (MV, RR). Criteria used for the assessment were those for seizures with generalized onset (e.g., bilateral uncontrolled movements, tonic posture, and/or loss of consciousness) and seizures with focal onset (e.g., hallucinations or cognitive deficits, and motor or sensory symptoms with evidence of seizure progression), and classified with the 2014 and 2017 definitions of the International League Against Epilepsy (i.e., ILAE) [21, 22]. Epilepsy was also defined according to ILAE guidelines [21, 22]. In case of doubt, events were not classified as epileptic seizures. We did not routinely use an electroencephalogram (EEG), we diagnosed seizures purely clinically. As a cut-off point for early seizures (ES) we used seven days, and after seven days we classified seizures as late seizures (LS) [23]. In case patient had both early and late seizures they would be defined as recurrent seizures, but these were analyzed as late seizures to maintain sample size.
We obtained the following variables: age at onset of the hemorrhage, gender, prior cerebrovascular disease (i.e., either ICH, ischemic stroke, or transient ischemic attack), vascular comorbidities (i.e., coronary heart disease, hypertension, diabetes mellitus, hypercholesterolemia, peripheral vascular disease, as determined by previous diagnosis in hospital records), National Institute of Health Stroke Scale at admission (NIHSS below 8, NIHSS 8-14, NIHSS above 14; grouped as described by Adams et al. [24]), location of hemorrhage (i.e., lobar, deep or infratentorial), septum shift, use of anticoagulants/antiplatelet drugs (i.e., either one, or combinational therapy), and recurrence of hemorrhage during follow-up (i.e., a second hemorrhage was not recorded as new hemorrhage). Furthermore, prior statin use, use of anti-epileptic drugs (AEDs), number of seizures before and after start of AEDs, and response to AED therapy were obtained. Municipal inhabitant registry was used to record all-cause mortality status for all patients, which is a very reliable method in the Netherlands to assess mortality as all deceased are registered by medical professionals, with the latest search dating March 14th 2016.
Statistical analyses
Pearson Chi-square tests were performed to assess differences amongst seizure groups (no seizures, ES, or LS) in categorical data, as was done for continuous data with the use of the Student’s t test for normally distributed data and the Mann–Whitney U test for non-normally distributed data.
Mortality in the different seizure groups was analyzed with Kaplan–Meier analyses and log-rank tests, whereas we used Cox proportional hazard’s multivariable analysis to assess independent predictors of mortality. All variables were univariately analyzed and included in the multivariate analyses when significant or previously proven relevant. Cox multivariate analyses were not only performed for the complete follow-up, but also for fixed intermediate intervals (i.e., 2.5, 5, 7.5, and 10 years after onset of hemorrhage). This was done to analyze the association between seizure development and mortality over time (i.e., whether seizures or other predictors are variably associated with altered survival rates for different years after ICH). We censored patients that did not reach the primary endpoint (mortality) before the last follow-up, dating March 14th 2016 (end-of-study censoring). Association measures were expressed as hazard ratios (HR), with 95% confidence intervals. p values are provided for indication of significance, set at a value of p ≤ 0.05. All statistical methods were performed using SPSS (SPSS inc. Version 23.0, Chicago, Illinois).
Results
Participants
We identified 1214 ICH patients in this retrospective study, and entered these into the selection process (Fig. 1). We excluded 393 patients who died within seven days after onset of the hemorrhage, 40 because of prior history of epilepsy, and 34 because of missing data: cerebrovascular disease (n = 1), cardiovascular disease (n = 8), hypertension (n = 9), diabetes (n = 5), hypercholesterolemia (n = 15), peripheral vascular disease (n = 11), and use of anticoagulants (n = 9) (Fig. 1). The remainder of 747 patients were used for all analyses. 641 (86%) patients did not develop seizures, 32 (4%) developed only early seizures, whereas 74 (10%) developed late seizures. Of these 74 patients, 29 had developed early seizures previously. These patients with recurrent seizures were analyzed as late seizures to maintain sample size. EEG-confirmation was available in 26 (20%) patients with late seizures, but none of the early seizures were EEG confirmed. Patients that developed late seizures mostly received AED therapy (93%). The follow-up for seizures and survival status had a median duration of 4.8 years (interquartile range of 0–12.6 years). Baseline characteristics of the study population are shown in Table 1.
Selection process as applied in the study. Exclusion was based on mortality within the first 7 days, and second, on missing data of medical history, given per variable. The final population was divided in three groups, group sizes given. ICH intracerebral hemorrhage, stroke both ischemic as hemorrhagic, CVD cardiovascular disease, HT hypertension, DM diabetes mellitus type 2, PVD peripheral vascular disease, Ac/Ap anticoagulants/antiplatelet drugs, n number of patients either excluded or still present in the study, NS no seizures, ES early seizures, LS late seizures
Outcome data
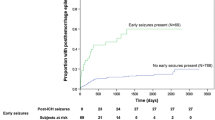
During follow-up, 465 (62%) patients died. 400 out of 641 (62%) died in the NS group, 20 out of 32 (54%) in the ES group, and 45 out of 74 (61%) in the LS group. See Fig. 2 for the Kaplan–Meier plots. The cumulative survival did not significantly differ between NS, ES, or LS (Log-rank p value 0.58).
Predictors of post-hemorrhage mortality
We found that relevant predictors of mortality after ICH were higher age, male gender, peripheral vascular disease, higher NIHSS scores, and use of anticoagulants (i.e., no differences between anticoagulants or antiplatelet drugs were found) (Table 2). Factor is no longer significant in any time interval if statin use is included in the analyses. An additional significant predictor of mortality, only in the long term (i.e., 7.5 and 10 years), was diabetes mellitus (Table 2).
Interestingly, amongst patients with late seizures, the use of AEDs did relate to lower mortality rates in the 7.5- and 10-year intervals [HR 0.32 (0.11–0.91)]. In this group, there was no relation between mortality and the number of seizures before initiation of medication, and reaction to anti-epileptic drugs. However, the number of patients with LS at this long follow-up interval was relatively low (n = 35 and n = 7 for 7.5 and 10 years, respectively).
Discussion
Our study underlines that seizures after ICH do not independently relate to higher late mortality risks after follow-up up to 10 years. Mortality was associated with higher age, male gender, peripheral vascular disease, higher NIHSS scores, and anticoagulant/antiplatelet drug use. Mortality in the long term (i.e., 10 years) was also determined by diabetes mellitus. Overall, we found that 10-year survival after ICH was 37% in our population, which was not significantly influenced by development of early or late seizures.
These results are in line with most [6, 10 13–15, 18], but not all [12, 16, 17] studies. These studies showed an increased or decreased risk of mortality in patients with seizures. Take note that these study populations were composed of both ischaemic stroke (IS) and ICH patients, and extrapolation should be done carefully though no differences between IS and ICH were mentioned. However, these previous studies did have significantly shorter periods of follow-up, and none of these studies found seizures to be an independent risk factor of mortality in multivariable analyses [12, 16, 18]. The one study that did indicate a significant independent relation, and showed a higher mortality rate in the seizure group, assessed a heterogeneous study population of supposedly vascular events (including ischemic stroke, transient ischemic attack and ICH) at a young age. However, authors did not specifically include ICH patients, and they did not exclude patients not surviving the first seven days after stroke [17]. Up and till now, studies with a long-term follow-up, specifically for ICH patients, are lacking to the best of our knowledge. Therefore, our study in ICH patients with a large sample size, long-term follow-up, and a selection process designed to study the long-term relation between seizures and mortality adds significantly to the existing reports. Our finding that mortality is not influenced by seizure development, but is mostly determined by age, stroke severity, and vascular risk factors might imply that patients may benefit more from management of these factors, than from management of the seizures.
AED use did correlate, independently from seizure frequency, with lower mortality risks in patients with late seizures 7.5 years after hemorrhage. This correlation weakened in the 10-year interval and disappeared in the full follow-up, probably due to the limited number of patients that already reached that time point. This might suggest that AED therapy improves long-term survival after ICH, though not likely due to lowered seizures frequencies. There are, however, some caveats, such as the small number of patients with late seizures that did not receive AED therapy (n = 5). Also, discrepancies in patient treatment may play a role in this as patients with worse clinical conditions might not have been treated equally, or even have not been treated at all. Though, Gilad et al. found a better neurological outcome after treatment with valproate in patients surviving an ICH, which, in combination with our findings, suggests a (weakly) positive effect of AED on recovery and prognosis after ICH [25]. This finding suggests that survival rates might benefit from AED therapy, but further research is needed to provide sufficient evidence.
Our study has some limitations. First, despite our large population, only 14% of participants developed seizures, which somewhat limits the power of this study. Also, there is bias due to the retrospective design, though efforts have been made to reduce the impact: we minimized the amount of missing data using diagnosis codes and hospital stroke registries. Furthermore, defining seizures retrospectively are another possible source of bias. Patients may underreport focal seizures or may not seek medical attention or overreport, for example, syncopes as seizures. Furthermore, subclinical seizures are missed because EEG monitoring was not routinely performed, which might lead to underestimation of the frequency of seizure development. As a consequence of the retrospective design, only survival status could be monitored in the period of follow-up after hospital discharge. Details on seizure severity and post-hospitalization comorbidities, which could underlie the association between AED therapy and decreased mortality, are not known. Furthermore, patients with worse prognosis might not have been treated at all. As all of these data are not available, these results have to be interpreted with caution.
Notwithstanding these limitations, the strengths of our study remain the large sample size allowing enough patients to develop early or late seizures, combined with the follow-up allowing sufficient time to develop late seizures and determine the long-term survival rates. Also, exclusion of patients who deceased during the first seven days allows to draw conclusions concerning the mortality risk based on seizure development, without interference of mortality risks primarily due to the hemorrhage itself.
In conclusion, seizure development does not relate to the mortality risk after ICH in our population. However, mortality mostly relates to age, stroke severity and vascular comorbidities and anticoagulant/antiplatelet use, and therefore, these prognostic factors are of value when discussing treatment and prognosis after ICH.
References
Ikram MA, Wieberdink RG, Koudstaal PJ (2012) International epidemiology of intracerebral hemorrhage. Current Atheroscler Rep 14(4):300–306
Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA et al (2014) Global and regional burden of stroke during 1990–2010: findings from the global burden of disease study 2010. Lancet 383(9913):245–254
Sacco S, Marini C, Toni D, Olivieri L, Carolei A (2009) Incidence and 10-year survival of intracerebral hemorrhage in a population-based registry. Stroke 40(2):394–399
Flaherty ML, Haverbusch M, Sekar P, Kissela B, Kleindorfer D, Moomaw CJ et al (2006) Long-term mortality after intracerebral hemorrhage. Neurology 66(8):1182–1186
Myint PK, Staufenberg EF, Sabanathan K (2006) Post-stroke seizure and post-stroke epilepsy. Postgrad Med J 82(971):568–572
Ferro JM, Pinto F (2004) Poststroke epilepsy: epidemiology, pathophysiology and management. Drugs Aging 21(10):639–653
Biffi A, Rattani A, Anderson CD, Ayres AM, Gurol EM et al (2016) Delayed seizures after intracerebral hemorrhage. Brain 139:2694–2705
Zhang C, Wang X, Wang Y, Zhang JG, Hu W, Ge M et al (2014) Risk factors for post-stroke seizures: a systematic review and meta-analysis. Epilepsy Res 108(10):1806–1816
Kammersgaard LP, Olsen TS (2005) Poststroke epilepsy in the Copenhagen stroke study: incidence and predictors. J Stroke Cerebrovasc Dis. 14(5):210–214
Menon B, Shorvon SD (2009) Ischaemic stroke in adults and epilepsy. Epilepsy Res 87(1):1–11
Agrawal A, Timothy J, Pandit L, Manju M (2006) Post-traumatic epilepsy: an overview. Clin Neurol and Neurosurg 108(5):433–439
Szaflarski JP, Rackley AY, Kleindorfer DO, Khoury J, Woo D, Miller R et al (2008) Incidence of seizures in the acute phase of stroke: a population-based study. Epilepsia 49(6):974–981
Hamidou B, Aboa-Eboule C, Durier J, Jacquin A, Lemesle-Martin M, Giroud M et al (2013) Prognostic value of early epileptic seizures on mortality and functional disability in acute stroke: the Dijon Stroke Registry (1985–2010). J Neurol 260(4):1043–1051
Mullen MT, Kasner SE, Messe SR (2013) Seizures do not increase in-hospital mortality after intracerebral hemorrhage in the nationwide inpatient sample. Neurocrit Care 19(1):19–24
Zelano J, Lundberg RG, Baars L, Hedegard E, Kumlien E (2015) Clinical course of poststroke epilepsy: a retrospective nested case-control study. Brain Behav 5(9):e00366
Burneo JG, Fang J, Saposnik G (2010) Investigators of the Registry of the Canadian Stroke Network. Impact of seizures on morbidity and mortality after stroke: a Canadian multi-centre cohort study. Eur J Neurol 17(1):52–58
Arntz RM, Rutten-Jacobs LC, Maaijwee NA, Schoonderwaldt HC, Dorresteijn LD, van Dijk EJ et al (2015) Poststroke epilepsy is associated with a high mortality after a stroke at young age: follow-up of transient ischemic attack and stroke patients and unelucidated risk factor evaluation study. Stroke 46(8):2309–2311
Reith J, Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS (1997) Seizures in acute stroke: predictors and prognostic significance. The copenhagen stroke study. Stroke 28(8):1585–1589
Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ et al (2014) Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Int J Surg 12(12):1500–1524
de Greef BT, Schreuder FH, Vlooswijk MC, Schreuder AH, Rooyer FA, van Oostenbrugge RJ et al (2015) Early seizures after intracerebral hemorrhage predict drug-resistant epilepsy. J Neurol 262(3):541–546
Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE et al (2014) ILAE official report: a practical clinical definition of epilepsy. Epilepsia 55(4):475–482
Fisher RS, Cross JH, D’Souza C, French JA, Haut SR et al (2017) ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia 58(4):512–521
Guidelines for epidemiologic studies on epilepsy, Commission on epidemiology and prognosis (1993) International league against epilepsy. Epilepsia 34(4):592–596
Adams HP Jr, Leclerc JR, Bluhmki E, Clarke W, Hansen MD, Hacke W (2004) Measuring outcomes as a function of baseline severity of ischemic stroke. Cerebrovasc Dis 18(2):124–129
Gilad R, Boaz M, Dabby R, Sadeh M, Lampl Y (2011) Are post intracerebral hemorrhage seizures prevented by anti-epileptic treatment? Epilepsy Res 95(3):227–231
Author information
Authors and Affiliations
Contributions
DC, acquisition of data, analysis and interpretation of data and drafting of manuscript. KB, acquisition of data, interpretation of data, and drafting of manuscript. FS, acquisition of data and critical revision of manuscript for intellectual content. BG, acquisition of data and critical revision of manuscript for intellectual content. MV, acquisition of data and critical revision of manuscript for intellectual content. JS, acquisition of data and critical revision of manuscript for intellectual content. RO, acquisition of data and critical revision of manuscript for intellectual content. RR, study concept and design, acquisition of data, interpretation of data, study supervision, and critical revision of manuscript for intellectual content
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare to have no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Claessens, D., Bekelaar, K., Schreuder, F.H.B.M. et al. Mortality after primary intracerebral hemorrhage in relation to post-stroke seizures. J Neurol 264, 1885–1891 (2017). https://doi.org/10.1007/s00415-017-8573-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-017-8573-1