Abstract
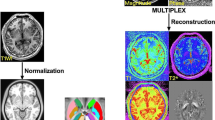
Vascular parkinsonism is a difficult clinical differential diagnosis in elderly subjects. We aimed at identifying morphometric markers in the brain of elderly patients with vascular parkinsonism (VP) compared with age-matched patients with Parkinson’s disease (PD) and healthy controls. In this multicenter prospective study, 46 patients (80 ± 5 years old; male 32) with parkinsonism (32 PD and 14 VP) and 29 controls (mean age 78 ± 3 years; male 21) underwent brain MRI on a 3-T scanner including T1 MPRAGE and FLAIR sequences. Volumetric morphometry was obtained using Morphobox software and compared between patients and controls. Receiver operating characteristics curve analysis with computation of area under the curve (AUC) was used to compare diagnostic values. Caudate nucleus and white matter hyperintense lesions (WMHL) volumes appeared significantly higher in patients with VP. Normalized caudate volume of at least 0.67% and normalized WMHL of at least 1.11% identified patients with VP from patients with PD and controls with similar performances (p > 0.25). Caudate nucleus and WMHL volumes were positively correlated (ρ = 0.74, p < 0.0001), suggesting vascular disease related remodelling in elderly subjects. Caudate nucleus and WMHL MRI volumes might be used as additional markers to help identify patients with VP in the initial workup of elderly subjects with parkinsonian symptoms.



Similar content being viewed by others
References
Vale TC, Caramelli P, Cardoso F (2015) Clinicoradiological comparison between vascular parkinsonism and Parkinson’s disease. J Neurol Neurosurg Psychiatry 86(5):547–553. doi:10.1136/jnnp-2014-307867
Benitez-Rivero S, Lama MJ, Huertas-Fernandez I, Alvarez de Toledo P, Caceres-Redondo MT, Martin-Rodriguez JF, Carrillo F, Carballo M, Palomar FJ, Mir P (2014) Clinical features and neuropsychological profile in vascular parkinsonism. J Neurol Sci 345(1–2):193–197. doi:10.1016/j.jns.2014.07.046
Korczyn AD (2015) Vascular parkinsonism—characteristics, pathogenesis and treatment. Nat Rev Neurol 11(6):319–326. doi:10.1038/nrneurol.2015.61
Cherubini A, Morelli M, Nistico R, Salsone M, Arabia G, Vasta R, Augimeri A, Caligiuri ME, Quattrone A (2014) Magnetic resonance support vector machine discriminates between Parkinson disease and progressive supranuclear palsy. Movement disorders: official journal of the Movement Disorder Society 29(2):266–269. doi:10.1002/mds.25737
Choi SM, Kim BC, Nam TS, Kim JT, Lee SH, Park MS, Kim MK, de Leon MJ, Cho KH (2011) Midbrain atrophy in vascular Parkinsonism. Eur Neurol 65(5):296–301. doi:10.1159/000326907
Focke NK, Helms G, Scheewe S, Pantel PM, Bachmann CG, Dechent P, Ebentheuer J, Mohr A, Paulus W, Trenkwalder C (2011) Individual voxel-based subtype prediction can differentiate progressive supranuclear palsy from idiopathic Parkinson syndrome and healthy controls. Hum Brain Mapp 32(11):1905–1915. doi:10.1002/hbm.21161
Kim YH, Ma HI, Kim YJ (2015) Utility of the Midbrain Tegmentum Diameter in the Differential Diagnosis of Progressive Supranuclear Palsy from Idiopathic Parkinson’s Disease. J Clin Neurol 11(3):268–274. doi:10.3988/jcn.2015.11.3.268
Menke RA, Szewczyk-Krolikowski K, Jbabdi S, Jenkinson M, Talbot K, Mackay CE, Hu M (2014) Comprehensive morphometry of subcortical gray matter structures in early-stage Parkinson’s disease. Hum Brain Mapp 35(4):1681–1690. doi:10.1002/hbm.22282
Pereira JB, Ibarretxe-Bilbao N, Marti MJ, Compta Y, Junque C, Bargallo N, Tolosa E (2012) Assessment of cortical degeneration in patients with Parkinson’s disease by voxel-based morphometry, cortical folding, and cortical thickness. Hum Brain Mapp 33(11):2521–2534. doi:10.1002/hbm.21378
Zijlmans JC, Thijssen HO, Vogels OJ, Kremer HP, Poels PJ, Schoonderwaldt HC, Merx JL, van’t Hof MA, Thien T, Horstink MW (1995) MRI in patients with suspected vascular parkinsonism. Neurology 45(12):2183–2188
Rampello L, Alvano A, Battaglia G, Raffaele R, Vecchio I, Malaguarnera M (2005) Different clinical and evolutional patterns in late idiopathic and vascular parkinsonism. J Neurol 252(9):1045–1049. doi:10.1007/s00415-005-0811-2
Demirkiran M, Bozdemir H, Sarica Y (2001) Vascular parkinsonism: a distinct, heterogeneous clinical entity. Acta Neurol Scand 104(2):63–67
Dunet V, Deverdun J, Charroud C, Le Bars E, Molino F, Menjot de Champfleur S, Maury F, Charif M, Ayrignac X, Labauge P, Castelnovo G, Pinna F, Bonafe A, Geny C, Menjot de Champfleur N (2016) Cognitive Impairment and Basal Ganglia Functional Connectivity in Vascular Parkinsonism. AJNR Am J Neuroradiol 37(12):2310–2316. doi:10.3174/ajnr.A4889
Vizcarra JA, Lang AE, Sethi KD, Espay AJ (2015) Vascular Parkinsonism: deconstructing a syndrome. Movement disorders: official journal of the Movement Disorder Society 30(7):886–894. doi:10.1002/mds.26263
Korczyn AD (2015) Vascular parkinsonism-characteristics, pathogenesis and treatment. Nature reviews Neurology 11(6):319–326. doi:10.1038/nrneurol.2015.61
Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, Lijmer JG, Moher D, Rennie D, de Vet HC, Kressel HY, Rifai N, Golub RM, Altman DG, Hooft L, Korevaar DA, Cohen JF, Group S (2015) STARD 2015: an Updated List of Essential Items for Reporting Diagnostic Accuracy Studies. Radiology 277(3):826–832. doi:10.1148/radiol.2015151516
Gibb WR, Lees AJ (1989) The significance of the Lewy body in the diagnosis of idiopathic Parkinson’s disease. Neuropathol Appl Neurobiol 15(1):27–44
Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, Goetz CG, Golbe LI, Grafman J, Growdon JH, Hallett M, Jankovic J, Quinn NP, Tolosa E, Zee DS (1996) Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 47(1):1–9
Zijlmans JC, Daniel SE, Hughes AJ, Revesz T, Lees AJ (2004) Clinicopathological investigation of vascular parkinsonism, including clinical criteria for diagnosis. Movement disorders: official journal of the Movement Disorder Society 19(6):630–640. doi:10.1002/mds.20083
Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N, Movement Disorder Society URTF (2008) Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 23(15):2129–2170. doi:10.1002/mds.22340
Schmitter D, Roche A, Marechal B, Ribes D, Abdulkadir A, Bach-Cuadra M, Daducci A, Granziera C, Kloppel S, Maeder P, Meuli R, Krueger G, Alzheimer’s Disease Neuroimaging I (2015) An evaluation of volume-based morphometry for prediction of mild cognitive impairment and Alzheimer’s disease. Neuroimage Clin 7:7–17. doi:10.1016/j.nicl.2014.11.001
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44(3):837–845
Yamanouchi H, Nagura H (1997) Neurological signs and frontal white matter lesions in vascular parkinsonism. A clinicopathologic study. Stroke J Cereb Circ 28(5):965–969
Caplan LR (2015) Lacunar infarction and small vessel disease: pathology and pathophysiology. J Stroke 17(1):2–6. doi:10.5853/jos.2015.17.1.2
Abela E, Seiler A, Missimer JH, Federspiel A, Hess CW, Sturzenegger M, Weder BJ, Wiest R (2014) Gray matter volumetric changes related to recovery from hand paresis after cortical sensorimotor stroke. Brain Struct Funct 220(5):2533–2550. doi:10.1007/s00429-014-0804-y
Prins ND, van Straaten EC, van Dijk EJ, Simoni M, van Schijndel RA, Vrooman HA, Koudstaal PJ, Scheltens P, Breteler MM, Barkhof F (2004) Measuring progression of cerebral white matter lesions on MRI: visual rating and volumetrics. Neurology 62(9):1533–1539
Acknowledgements
The authors would like to thank all subjects who participated to this study as well as Benedicte Marechal for her advices and technical assistance in using Morphobox software. This work was supported by grants from the CHRU of Montpellier, which was not involved in any step of the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare they have no conflict of interest regarding this study.
Ethical standard
This study was approved by the Institution Ethics Committee and has, therefore, been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
All participants gave their informed consent prior to their inclusion in the study. There are no details in this manuscript that might disclose the identity of the participants.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dunet, V., Deverdun, J., Charroud, C. et al. MRI volumetric morphometry in vascular parkinsonism. J Neurol 264, 1511–1519 (2017). https://doi.org/10.1007/s00415-017-8561-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-017-8561-5