Abstract
Driving is important for employment, social activities, and for the feeling of independence. The decision to cease driving affects the quality of life and has been associated with reduced mobility, social isolation, and sadness. Patients with neurodegenerative disorders can experience difficulties while driving due to their cognitive, motor, and behavioral impairments. The aim of this review is to summarize the available literature on changes in driving competence and behavior in patients with neurodegenerative disorders, with a particular focus on Huntington’s (HD), Parkinson’s (PD), and Alzheimer’s disease (AD). A systematic literature search was conducted in the PubMed/Medline database. Studies using on-road or simulated driving assessments were examined in this review. In addition, studies investigating the association between cognitive functioning and driving were included. The review identified 70 studies. Only a few publications were available on HD (n = 7) compared to PD (n = 32) and AD (n = 31). This review revealed that driving is impaired in patients with neurodegenerative disorders on all levels of driving competence. The errors most commonly committed were on the tactical level including lane maintenance and lane changing. Deficits in executive functioning, attention, and visuospatial abilities can partially predict driving competence, and the performance on neuropsychological tests might be useful when discussing potential driving cessation. Currently, there is no gold standard to assess driving ability using clinical measures such as neuropsychological assessments, so more studies are necessary to detect valid screening tools and develop useful and reliable evidence-based guidelines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Progressive neurodegenerative diseases can result in a loss of motor and cognitive functioning, which interfere with daily activities such as the ability to drive a car [1]. Many individuals rely on their car for employment, social activities, and independency [2–4]. Therefore, the decision to cease driving affects the quality of life. Driving cessation has been associated with negative outcomes such as social isolation, reduced mobility, and sadness [5]. A difficult question that clinicians face in everyday practice is when to advise patients with early disease to abstain from driving. In most European countries, neurologists evaluate driving competence in patients with neurodegenerative disorders, based on their clinical examination [6]. Depending on the outcome of this evaluation, patients can be advised to contact an official national driving evaluation center. However, the evaluations of neurologists are often an overestimation of the actual driving capacities and inconsistent with on-road performances [3]. In the Netherlands, a neurologist has to evaluate if a patient should perform a formal driving test [7]. However, the decision to inform the national driving evaluation center relies on the self-report of patients. If a patient passes the formal driving test, the driver license can be renewed with a maximum of 5 years. Within this 5-year period, patients have no obligation to perform a retest. This can potentially be unsafe with the progressive character of neurodegenerative diseases, especially since changes in cognitive and daily functioning can already occur within 5 years [8, 9].
The aim of this review is to provide an overview of the available literature on changes in driving competence in patients with neurodegenerative disorders and to identify potential gaps in the literature that should be further investigated, with particular interest for Huntington’s disease (HD), Parkinson’s disease (PD), and Alzheimer’s disease (AD). We focused on these neurodegenerative disorders, since they are comparable in cognitive, psychiatric, and motor symptoms. A comprehensive review incorporating all three diseases has not been published before. Furthermore, we evaluate if specific cognitive tests have been identified that are predictive of driving ability and if these tests can be implemented in the clinical practice. Since simulators are increasingly being used in driving research and might be a proper screening tool to assess driving in patients with neurodegenerative diseases, we also included available literature on driving simulators.
Methods
An electronic database search in PubMed/MEDLINE was performed to identify the available literature. The last database search was performed on 27th October 2016. The following search terms were used individually and in combination: “driving” “driving ability” “neurodegeneration”, “Huntington’s disease”, “Huntington”, Parkinson’s disease”, “Parkinson”, “Alzheimer’s disease”, “Alzheimer”, “dementia”, “cognition”, “cognitive functioning”, and “simulator”. In addition, references and reviews were checked in search of relevant studies. In the initial search, only papers written in English were considered and selected for further review. Only original articles and full communications were included (e.g., no letters to editors, editorial comments, or reviews). Articles were deemed relevant if they directly investigated driving-related issues using formal driving assessments (i.e., on-road or simulator) in diagnosed patients with HD, PD, or AD.
Results
Search results
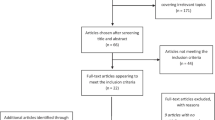
The database search yielded 240 articles that were selected for further review based on title. The abstract of each article was reviewed and the inclusion/exclusion criteria were checked. From these 240 articles, 70 studies met the inclusion criteria of the current review (7 HD, 32 PD, and 31 AD studies). The majority of the studies described on-road driving performances (n = 45), 21 studies involved driving simulation, and 51 articles investigated the relationship between cognitive performances and driving outcomes. A summary of the included literature and the methods that were used is given per group in Tables 1, 2, and 3. When applicable, we will use the driving model of Michon et al. [10]. According to this model, driving errors can be sorted in three categories: (a) strategic errors that occur before actual driving, such as route planning; (b) tactical errors consisting of errors in speed adaptations, changing lanes, and keeping distance; (c) operational errors such as incorrect responses to changing driving environments and vehicle control [11, 12]. An overview of the committed driving errors by patient group per category is given in Table 4.
Driving and Huntington’s disease
Huntington’s disease (HD) is a hereditary neurodegenerative disorder characterized by choreatic movements, cognitive dysfunction, and psychiatric symptoms [13]. It is caused by a gene mutation located on chromosome 4 [14]. The mean age at onset is between 30 and 50 years, with a mean disease duration of 17–20 years [13]. The earliest cognitive symptoms are characterized by executive dysfunctions, such as difficulties in planning, cognitive inflexibility, and lack of awareness [13, 15]. The cognitive symptoms gradually worsen and eventually result in dementia. Due to the progressive nature of the disease, patients become more dependent in their daily life activities. With the onset of HD during midlife, a lot of patients rely on their car for work and social activities, so patients might find it difficult to decide when to stop driving. However, concern about driving safely is one of the first issues reported by HD patients (33.5%) and has been associated with motor, cognitive, and depressive symptoms [16, 17]. The influence of other psychiatric symptoms, such as aggression and impulsivity, has not yet been investigated.
Only seven studies were found that investigated driving in HD patients [16–22]. Four of these studies used formal driving assessments, either on-road or simulated, to investigate driving competence [18, 20–22]. Due to the limited amount of studies available on HD and driving, the studies that did not investigate driving with formal driving assessments but with questionnaires or retrospective data analyses are also discussed [16, 17, 19]. An observational study investigating the association between different disease aspects of HD with functional changes showed that motor functioning and the Stroop task, measuring cognitive flexibility and information processing, were significantly associated with driving safety [16]. Increased motor impairment was related to a lower likelihood of being able to drive safely as rated by a professional. This study did not include a formal driving assessment. During a semi-structured interview, 11 out of 16 HD participants reported changes in their driving behavior [17]. They reported lower reaction times, had concerns about their safety, and had difficulties multi-tasking. A study that investigated clinical predictors of driving by retrospective patient file reviews showed that cognitive impairment, especially a reduction of psychomotor speed and attention, is a strong risk factor for driving cessation in HD [19]. Increased motor impairments were also associated with not driving a car, but were not a risk factor affecting the decision to cease driving [19]. An early study investigating driving in HD with a driving simulator showed that HD patients committed errors on the operational and tactical level [18]. They were less accurate and had longer reaction times compared to controls [18]. HD patients also had higher error rates in signaling, steering, braking, maintaining speed, and accelerator use. They were more likely to be involved in accidents compared to healthy individuals (58 and 11% respectively) [18]. Still, most of the HD patients in this study continued driving after onset of the disease (53/73). In addition, half of the HD patients that still drive failed an on-road driving assessment [20]. This confirms a limited insight regarding their own driving skills and emphasizes the importance of early evaluation [23–25]. In one study, 14 of the 30 HD patients (47%) failed the on-road driving test [21]. HD patients committed most errors on the operational and tactical levels, including errors in lane positioning, speed adaptations, keeping distance, turning left, and lane changing [21]. They also made more errors in perception of road signs, reflecting errors on the strategic level. Selective attention and disease stage were highly correlated with on-road driving failure in manifest HD [21]. A combination of neuropsychological tasks measuring visual processing speed, visual scanning, and attentional shifting best predicted the pass/fail rate of an on-road driving assessment, instead of a model that also included motor functioning [20]. More recently, it has been reported that some neuropsychological assessments focusing on speed of processing, cognitive flexibility, and visual attentional control seem to be good predictors for driving competence in manifest HD [22].
The results of the reviewed studies showed that driving competence is impaired in patients with HD and that concerns about driving safely are one of the earliest symptoms reported by both patients and families. Especially executive functioning and visuospatial abilities have been related to driving competence in HD. However, due to the limited amount of data, no conclusions can be drawn regarding which cognitive battery is most predictive of driving impairment in HD. None of the studies to date have focused on evaluating driving competency in the earliest stages of HD or in gene mutation carriers without a clinical diagnosis (i.e., premanifest gene carriers), while they often have questions for their physician regarding their driving skills and are most likely in need of a driving evaluation in the near future. Furthermore, no longitudinal studies have been performed investigating driving in HD, so there are no results available about the potential decline in driving competence during the course of the disease. Follow-up measurements are important to determine when driving-related issues become apparent and when to discuss potential driving cessation. It also provides an opportunity to monitor driving from early to more advanced stages of the disease.
Driving and Parkinson’s and Alzheimer’s disease
Contrary to driving studies in HD, a large number of studies have been performed evaluating driving competence in Parkinson’s disease (PD; n = 32) and Alzheimer’s disease (AD; n = 31). Three studies compared the driving competence of patients with PD and AD. In the following sections, we will discuss the on-road driving studies first, followed by the studies using driving simulators, and last the studies that also incorporated cognitive functioning in relation to driving performance.
Parkinson’s disease
Studies using on-road driving assessments (n = 22) to evaluate driving competence showed that 12–56% of the PD patients failed an on-road driving test [1, 26–34]. PD patients had a higher number of total driving safety errors compared to control participants. Studies that focused on identifying specific driving errors showed that PD patients are most likely to make errors on a tactical level including difficulties with yielding at intersections [29] and lane changing [1]. They were less likely to check their blind spot, and used their rear view and side mirrors less frequently than controls [1, 35]. Patients with PD also showed a decreased awareness of others, hesitated longer before making a turn, did not accelerate to a proper speed, and were less concentrated [26]. They made more errors in adjusting to different driving situations compared to controls [29] and showed difficulties driving in traffic flow [3]. PD patients made more errors in reversing and car parking [1]. Drivers with PD also had more difficulties with road positioning and driving on roundabouts compared to controls [33]. Most of the errors were present while driving in an urban environment [3]. Errors in the lateral position on the road at low speed and turning left [3] were the best predictors of overall pass/fail driving outcome [32]. Overall, PD patients had an unsteady car speed and tended to drive slower [35–37], especially during distraction [38]. However, it has also been reported that they drove faster on highways compared to controls [37], and had more difficulties adapting their speed at a higher speed [32]. They also identified fewer traffic signs and landmarks compared to controls [39].
On the operational level, PD patients made more incorrect turns and did not signal appropriately compared to controls [26, 35, 36]. They also made more errors in lane maintenance [1, 29, 40]. Strategically, PD patients made fewer driving trips [37, 41], drove less distance, and shorter durations [1, 41] compared to controls. PD patients had a higher preference for driving with a passenger [1, 37], which reported less nighttime driving [29, 37] and more often used alternative transportation [29]. Driving simulator studies (n = 12) showed that patients with PD had lower reaction times [42, 43], missed more red lights, and showed impaired accuracy compared to control subjects [42]. Furthermore, they had a higher number of traffic offences [43], more accidents [43, 44], and a worse overall simulator score compared to controls [43]. Patients who passed an on-road driving assessment also performed better on the simulator tests compared to patients who failed the on-road assessment [31]. Patients with PD tended to drive faster than controls and had poorer vehicle control, especially during low contrast visibility conditions [45]. PD patients were found to brake later during incongruent driving conditions [46]. They waited for external cues before they responded, while control subjects initiated a response prior to the cue. This result is similar to another study which found that PD patients relied more on external than internal cues to regulate their driving behavior [47].
A number of studies have incorporated cognitive assessments in an attempt to determine which test performances are associated with the driving competence of patients with PD. Most studies reported an association between cognitive functioning and driving competence [3, 12, 26–28, 31, 32, 36, 38–40, 43, 46, 48–52]. However, some studies also reported no associations between cognition and driving in PD patients [1, 33, 53], so results are inconsistent. Driving errors were particularly associated with lower performances in cognitive flexibility [26, 27, 38, 39, 49, 52], visuoconstructional abilities [26, 36, 39], attention [12, 27, 32, 36, 40, 46], psychomotor speed [46, 51], working memory [12, 49], set shifting [12, 48], information processing [12, 49], contrast sensitivity [27, 31, 43, 48, 51], visual scanning [32], visual acuity [32, 40], speed of visual processing [3, 27, 28, 40], and visual memory [3, 36].
Alzheimer’s disease
Twenty-three studies were included in this review that investigated driving competence in AD using on-road driving tests. Between 15 and 65% of the AD patients failed an on-road driving assessment [54–64]. They had lower overall driving performance scores compared to controls and committed more overall driving errors [62, 65–71], even in situations that were not considered challenging [54]. Driving performance scores tended to decrease with increasing dementia [57, 63, 72]. The largest decline in driving performance was reported in mild AD patients [57].
On a tactical level, AD patients committed more errors compared to controls in lane positioning [54, 67, 73], lane changing [57, 74], and checking their blind spot [74], and they tended to drive slower [68, 75]. They also had a higher inability to stop the vehicle appropriately [54, 76], and more difficulties avoiding potential collisions compared to controls [76]. Errors in turning [54, 70, 73, 75, 77], signaling [57, 74], and lane maintenance [54, 67, 73] were the most reported errors on the operational level. In contrast, some studies showed no differences between AD patients and healthy individuals in vehicle control [54, 70]. Strategic errors included less attention while driving, slower decision-making, and difficulties with road rules compared to controls [54]. AD patients also had more planning difficulties [75], identified fewer landmarks and traffic signs compared to controls [71], and showed more problems with route following [70].
Comparing driving competence of patients with PD and AD using on-road driving assessments showed that both patient groups committed more overall driving errors compared to controls [73]. These driving errors increased when a concurrent task was included [73]. There are also differences reported between both groups in types of driving errors [74]. Both AD and PD patients committed most errors on the tactical level, but patients with AD also made errors on the operational and strategic levels. Patients with PD committed relatively few operational and strategic errors compared to AD patients [74]. AD patients reported fewer driving trips and drove less miles compared to patients with PD and controls [62, 74]. Contrary, minimal differences between both groups have also been reported [53, 73].
The nine simulator studies reviewed showed that AD patients committed more errors in lane keeping (i.e., more lane deviations) [64, 78–81], turning left [78], and vehicle control [80] compared to controls. AD patients also tended to drive slower [64, 78, 80], took longer to complete the driving tests [78, 79], had less brake pressure [78], and made more judgmental errors (e.g., accidents, collisions) [80]. They failed to stop at traffic lights [80, 81] and exceeded the speed limit more often than controls [81]. Six out of eighteen AD patients crashed during a simulator test [82]. Cognitive and visual tests were predictive of the number of crashes [81–83]. Contrary, no differences in number of crashes between AD patients and controls have also been reported [83]. AD patients performed best when single, simple auditory-only driving navigation instructions were used compared to visual plus audio or visual-only instructions [84].
Drivers with increased cognitive impairments were more likely to be unsafe drivers compared to control subjects [74]. AD patients who failed an on-road assessment performed worse on neuropsychological tasks compared to AD patients who passed the on-road test [64]. Decreased performances on cognitive tests measuring speed of processing [62, 67, 73, 85], executive functioning [56, 74], attention [56, 70–72, 76], memory [67, 68, 70, 71, 73, 76], set shifting [62, 71, 73], visuoconstructional and visuospatial abilities [56, 67, 70, 71, 73, 74, 76], visual searching [56, 67, 72], and visual tracking [68] have been associated with worse scores on driving outcome variables and increased error rates in patients with AD. A composite battery of tests was more predictive of driving than individual tests [60, 67], and cognitive performance was more predictive of driving ability than AD diagnosis alone [85]. However, no correlations between neuropsychological outcome measures and on-road evaluations have also been reported [58, 77].
Self-assessment of driving performances
In addition to differences in driving performances, there are also differences reported in the evaluation of driving ability performed by patients, caregivers, and physicians. One study reported that PD patients rated their own driving performances lower than controls [50]. Contrary results showed that about 20% of the PD and AD patients misjudged their own driving ability [3, 43]. In addition, the rating performed by a neurologist (M = 8.0) was more optimistic compared to the rating performed by a driving instructor (M = 5.1) and psychologist (M = 5.7) [3]. Spouses tended to overestimate the driving ability of AD patients [86]. Ratings performed by an adult child were more related to driving outcome variables than ratings performed by spouses [86]. Self-ratings of driving ability performed by AD patients and ratings by spouses were significantly higher than ratings by an independent evaluator and physician [65, 87]. Ratings by a clinician were poorly associated with an on-road driving test, but not with naturalistic driving [86]. However, these clinician ratings were still more associated with driving performance compared to the self-evaluation by patients and the evaluation by spouses [65]. Caregivers did acknowledge general problems with driving, but still rated the AD patients driving competence significantly higher than an independent rater [87].
Driving simulator use
Since on-road driving assessments in patients with neurodegenerative disorders might be unsafe, an alternative is to evaluate driving competence with a simulator. Driving simulators provide the opportunity to present challenging situations and events in a standardized setting, with a high reproducibility compared to on-road driving assessments where situations cannot be manipulated [88]. Simulators are also used to train novice drivers before they start their on-road driving lessons [89]. Results of a concurrent and discriminant validity study comparing an on-road driving assessment with driving simulator tasks revealed that a driving simulator is a valid measure of driving performance for research purposes [90]. The driving simulator outcomes were able to discriminate between drivers with different levels of experience. In a study with elderly drivers, over 65% of the variability in the on-road assessments could be explained by driving simulator outcomes [91]. Adding a driving simulator increased the total variance explained by a potential screening battery to 60 and 94% [31, 43], suggesting that a driving simulator might be a useful screening tool to evaluate driving fitness. Studies that described the use of simulators for rehabilitation and training purposes in various disorders showed promising results, with more patients passing an on-road assessment after training with a simulator [92]. The lower ecological validity of a simulator, however, could be a disadvantage, because participants may prefer driving a real vehicle. It is also important to keep in mind that a reduction of driving performance measured with a simulator might reflect the adaption to the simulator itself and not actual driving ability. Therefore, it is necessary to further investigate the differences between disease groups and healthy individuals to minimize the effects of simulator use. In addition, the relationship between on-road performances and simulator driving should be further explored to determine whether simulator outcome measures are, indeed, consistent with on-road driving performance.
A common issue in simulator research is the existence of simulator sickness, which is comparable to motion sickness [93, 94]. It includes dizziness, nausea, vomiting, and sweating. The symptoms of simulator sickness are typically less severe than motion sickness and tend to decrease with multiple exposure and time [94, 95]. Dropouts in simulator studies have been related to simulator sickness, with up to one-third of the participants experiencing signs of simulator sickness [64, 84, 91]. The duration and configuration of the driving scenario influence this dropout rate [96]. For example, scenarios including more turns and sudden stops increase the risk for simulator sickness. Older age, female gender, and prior history of motion sickness have also been associated with higher susceptibility of experiencing simulator sickness [97, 98]. However, dropouts are not necessarily those subjects with the poorest performances [98, 99]. Several theories have been proposed to explain the occurrence of simulator sickness [94]. A conflict between structures within the sensory and vestibular systems has been the most widely excepted theory [94, 100]. When using a simulator to evaluate driving competence, this side-effect should be taken into consideration by excluding patients who experience simulator sickness or by screening beforehand. However, this might result in selection bias that should be accounted for. For more information regarding the topic of simulator sickness, we refer to the systematic review by Classen et al. [97].
Discussion
The majority of studies investigated driving competence of patients with a neurodegenerative disorder with on-road driving assessments, and this is considered the gold standard. Results showed worse driving performances in patients compared to controls, although there is a large variability in types of driving errors. Most errors are committed in lane changing, lane maintenance, lower reaction times, and larger variabilities in speed. Inconsistencies in results might be attributable to different methods and outcome measures. In addition, there is a large heterogeneity in the patient population and sample sizes (range n = 16–266). Specific types of driving errors are often not investigated and only global pass/fail ratings are reported. For research purposes, it is important to determine which types of driving errors are most common and if these errors also pose a safety hazard for the patient and environment. Some errors might be manageable and do not necessarily mean that the patient should cease driving. For example, errors on the strategic level, such as difficulties with planning a route, are less dangerous and more manageable than errors concerning reacting to other road users and vehicle control. Adaptations to the vehicle might also increase the time that a patient is still able to drive safely. PD patients were better drivers when they used an automatic car compared to a manually operated car [34]. Driving simulators have the potential to assist in investigating driving competence, but there are still limited results available. In addition, there is the phenomenon of simulator sickness that should be considered when using a simulator [97]. There is also variability in types of driving simulators (i.e., manufacturers) and scenarios that are used. Driving simulator studies often use motorway scenarios, because they are less susceptible to simulator sickness. These scenarios are useful to investigate reaction times and speed adaptations, but might not properly reflect the driving ability on the road in an environment with more distractors. Driving scenarios including rural or urban areas, with more traffic, different speeding zones, and sudden events, might be more difficult due to the higher demand on cognitive functioning. The utility of a driving simulator to predict on-road driving behavior in both research and clinical practice has to be further explored.
In most studies, more than half of the patients with a neurodegenerative disorder were classified as safe drivers. This suggests that a majority of the patients can still drive safely. Therefore, professionals should not base their recommendations about potential driving cessation solely on the presence of a clinical diagnosis [85]. Individual evaluations are important and changes in driving performance should be monitored regularly, preferable every year. Due to the progressive nature of neurodegenerative disorders, formal retesting of driving skills is recommended even if the driver license has been renewed for an extended period of time. Although this is not a review on driving competence in the normal elderly population, the influence of aging should be taken into consideration. However, the mean ages in the reviewed studies were relatively young (HD = 43.1 years, PD = 66.4 years, AD = 74.0 years), and most analyses were corrected for the effects of age. This suggests that older age alone is not a criterion to continue or cease driving.
Overall, the findings reported in the reviewed studies suggest that cognitive functioning is associated with safely operating a vehicle. The current literature suggests some consensus on which cognitive domains are associated with decreased driving competence. Diminished functioning in the visuocontructional, visuospatial, executive, and attentional domains has consistently been associated with impaired driving. Specific neuropsychological assessments are partially predictive of driving outcomes, but there is currently no valid screening battery that can accurately be used in the clinical practice. There are limited cut-off scores available, so it is still difficult to translate performances on neuropsychological tests to clinical recommendations. The most promising screening batteries, with sensitivity and specificity ranging between 61 and 94%, included the Trail Making Test (TMT), useful field of view (UFOV), Pelli–Robson, and Symbol Digit Modalities Test (SDMT). Baseline and follow-up assessments are necessary to further validate the usefulness of these tests. Recently, it has been reported that a combination of assessments (i.e., clinical interviews, neuropsychological assessments, and driving simulator outcomes) best predicted fitness to drive in patients with AD [64]. Furthermore, composite neuropsychological test batteries have been more predictive of driving performances than separate tests [26, 31, 32, 40, 50, 60, 67]. This suggests that a composite battery including multiple cognitive domains might be a reliable predictor of driving performance. However, this approach should be further validated before the practical application of such a screening battery can be determined.
Our review showed that there is still a gap in the current driving literature. Only a limited amount of longitudinal studies have been performed in AD and PD but none in HD. Follow-up is important for early intervention and to monitor changes over time. Moreover, there is a large discrepancy in the amount of studies available regarding driving in HD compared to PD and AD. Since the etiology of HD is known, this disorder could potentially be a good prototype to investigate changes in driving competency and the association with cognitive decline. Furthermore, there is the opportunity to investigate both symptomatic and asymptomatic gene carriers in an attempt to detect at which point in the disease driving-related issues become apparent. This is particularly useful for the clinical practice and to establish guidelines for patients, families, and caregivers. An important factor differentiating HD from PD and AD is the age at onset. HD typically occurs during midlife with a mean age at onset between 30 and 50 years, while signs and symptoms of PD and AD are most often developed later in life [13, 101, 102]. With this relatively young age at onset of HD, most patients still rely on their car for employment and social activities. Therefore, discussing driving ability is important at an early stage of the disease. Furthermore, no studies have been performed regarding the association between psychiatric symptoms (e.g., irritability and apathy) and driving. These are important signs of HD that can already be present at early stages of the disease and might influence driving behavior [15].
Both HD and PD can be distinguished from AD by the presence of motor disturbances, but the nature of cognitive deficits also differs. The cognitive impairments observed in AD can be considered a cortical dementia, whereas HD and PD are mainly characterized by subcortical changes [103, 104]. In HD and PD, problems in the executive domain are most commonly observed, while in AD, memory impairments are more pronounced [105, 106]. This different expression of cognitive profiles might also affect driving in distinctive ways. In addition, specific subtypes of motor signs in PD (i.e., tremor versus dyskinesia) potentially influence the ability to operate a car. Differences between these specific subtypes in fitness to drive have not been studied to date. However, it has been reported that patients with postural instability and gait disorder PD subtype failed an on-road driving assessment more often than patients with the tremor dominant subtype of PD (46 versus 7%) [32]. Different motor subtypes can also be distinguished in HD (chorea versus hypokinesia-rigidity) and these subtypes have been associated with between different cognitive profiles [107, 108]. These differences in symptomatology should be further investigated in relation with driving performance to increase knowledge about important individual differences.
An important issue to keep in mind is the limited insight of patients with neurodegenerative disorders into their own disabilities. We believe that it is important to discuss driving in the outpatient clinic in the presence of spouses or relatives to ascertain a more objective point of view. However, some partners might find it difficult to express their concerns with the patient there. The role of the physicians is important to start the discussion at the right time and to provide the necessary referrals. On the same note, it is interesting to further explore the patient’s perspective regarding driving cessation, since some studies did report that there are patients who modify their driving behavior [109, 110].
In general, there are numerous difficulties in performing driving research in neurodegenerative disorders that should be considered when developing study protocols. An important issue is the presence of potential selection bias. Patients might fear that their license will be revoked and, therefore, do not want to participate in driving-related studies [111]. Patients who are less confident about their driving ability might be less willing to participate. In addition, there are safety concerns when evaluating driving performances. Other issues are the relatively small sample sizes, lack of control groups, and differences in methodology.
Conclusion
Based on the current available literature, it is not possible to draw one final conclusion if and when patients with neurodegenerative disorders should be restricted in their driving. Driving requires optimal cognitive functioning and lower performances on neuropsychological assessments might serve as a first indicator of driving incompetence. However, there is currently no validated screening battery available. Some patients with neurodegenerative disorders are still able to drive safely, so a restriction of driving solely based on a clinical diagnosis is unwarranted. None of the studies to date have resulted in practical guidelines that can be implemented in clinical settings. We are of the opinion that formal retesting should be mandatory due to the progressive nature of neurodegenerative diseases. Longitudinal studies are, therefore, necessary to determine when driving-related issues become apparent and to investigate the progression rate of driving incompetence. Further studies focusing on establishing specific evidence-based guidelines that take differences between disorders into consideration are needed. The lack of patient insight into their own driving competence should be further explored and emphasizes the need to quantify driving status.
References
Wood JM, Worringham C, Kerr G et al (2005) Quantitative assessment of driving performance in Parkinson’s disease. J Neurol Neurosurg Psychiatry 76:176–180
Adler G, Rottunda S (2006) Older adults’ perspectives on driving cessation. J Aging Stud 20:227–235
Heikkilä VM, Turkka J, Korpelainen J et al (1998) Decreased driving ability in people with Parkinson’s disease. J Neurol Neurosurg Psychiatry 64:325–330
Taylor BD, Tripodes S (2001) The effects of driving cessation on the elderly with dementia and their caregivers. Accid Anal Prev 33:519–528
Liddle J, Tan A, Liang P et al (2016) “The biggest problem we’ve ever had to face”: how families manage driving cessation in people with dementia. Int Psychogeriatr 28:109–122
White S, O’Neill D (2000) Health and relicensing policies for older drivers in the European union. Gerontology 46:146–152
CBR (2000) Regeling eisen geschiktheid. Retrieved October 17, 2016 from http://www.cbr.nl
Stout J, Jones R, Labuschagne I et al (2012) Evaluation of longitudinal 12 and 24 month cognitive outcomes in premanifest and early Huntington’s disease. J Neurol Neurosurg Psychiatry 83:687–694
Muslimović D, Post B, Speelman JD et al (2009) Cognitive decline in Parkinson’s disease: a prospective longitudinal study. J Int Neuropsychol Soc 15:426–437
Michon JA (1989) Explanatory pitfalls and rule-based driver models. Accid Anal Prev 21:341–353
Stolwyk RJ, Triggs TJ, Charlton JL et al (2006) Effect of a concurrent task on driving performance in people with Parkinson’s disease. Mov Disord 21:2096–2100
Stolwyk RJ, Charlton JL, Triggs TJ et al (2006) Neuropsychological function and driving ability in people with Parkinson’s disease. J Clin Exp Neuropsychol 28:898–913
Roos RAC (2010) Huntington’s disease: a clinical review. Orphanet J Rare Dis 5:1–8
The Huntington’s Disease Collaborative Research Group (1993) A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 72:971–983
Bates GP, Dorsey R, Gusella JF et al (2015) Huntington disease. Nat Rev Dis Primers 1:1–21
Beglinger LJ, O’Rourke JJF, Wang C et al (2010) Earliest functional declines in Huntington’s disease. Psychiatry Res 178:414–418
Williams JK, Downing NR, Vaccarino AL et al (2011) Self reports of day-to-day function in a small cohort of people with Prodromal and Early HD. PLoS Curr Huntingt Dis 1:1–13
Rebok GW, Bylsma FW, Keyl PM et al (1995) Automobile driving in Huntington’s disease. Mov Disord 10:778–787
Beglinger LJ, Prest L, Mills JA et al (2012) Clinical predictors of driving status in Huntington’s disease. Mov Disord 27:1146–1152
Devos H, Nieuwboer A, Tant M et al (2012) Determinants of fitness to drive in Huntington disease. Neurology 79:1975–1982
Devos H, Nieuwboer A, Vandenberghe W et al (2014) On-road driving impairments in Huntington disease. Neurology 82:956–962
Hennig BL, Kaplan RF, Nowicki AE et al (2014) We can predict when driving is no longer safe for people who have HD using standard neuropsychological measures. J Huntingt Dis 3:351–353
Hoth KF, Paulsen JS, Moser DJ et al (2007) Patients with Huntington’s disease have impaired awareness of cognitive, emotional, and functional abilities. J Clin Exp Neuropsychol 29:365–376
Sitek EJ, Thompson JC, Craufurd D, Snowden JS (2014) Unawareness of deficits in Huntington’s disease. J Huntingt Dis 3:125–135
McCusker E, Loy CT (2014) The many facets of unawareness in Huntington disease. Tremor Other Hyperkinet Mov 4:1–8
Amick MM, Grace J, Ott BR (2007) Visual and cognitive predictors of driving safety in Parkinson’s disease patients. Arch Clin Neuropsychol 22:957–967
Classen S, McCarthy DP, Shechtman O et al (2009) Useful field of view as a reliable screening measure of driving performance in people with Parkinson’s disease: results of a pilot study. Traffic Inj Prev 10:593–598
Classen S, Witter DP, Lanford DN et al (2011) Usefulness of screening tools for predicting driving performance in people with Parkinson’s disease. Am J Occup Ther 65:579–588
Classen S, Brumback B, Monahan M et al (2014) Driving errors in Parkinson’s disease: moving closer to predicting on-road outcomes. Am J Occup Ther 68:77–85
Crizzle AM, Classen S, Lanford DN et al (2013) Postural/gait and cognitive function as predictors of driving performance in Parkinson’s disease. J Parkinson’s Dis 3:153–160
Devos H, Vandenberghe W, Nieuwboer A et al (2013) Validation of a screening battery to predict driving fitness in people with Parkinson’s disease. Mov Disord 28:671–674
Devos H, Vandenberghe W, Tant M et al (2013) Driving and off-road impairments underlying failure on road testing in Parkinson’s disease. Mov Disord 28:1949–1956
Radford KA, Lincoln NB, Lennox G (2004) The effects of cognitive abilities on driving in people with Parkinson’s disease. Disabil Rehabil 26:65–70
Singh R, Pentland B, Hunter J, Provan F (2007) Parkinson’s disease and driving ability. J Neurol Neurosurg Psychiatry 78:363–366
Cordell R, Lee HC, Granger A et al (2008) Driving assessment in Parkinson’s disease—a novel predictor of performance? Mov Disord 23:1217–1222
Uc EY, Rizzo M, Anderson SW et al (2007) Impaired navigation in drivers with Parkinson’s disease. Brain 130:2433–2440
Crizzle AM, Myers AM (2013) Examination of naturalistic driving practices in drivers with Parkinson’s disease compared to age and gender-matched controls. Accid Anal Prev 50:724–731
Uc EY, Rizzo M, Anderson SW et al (2006) Driving with distraction in Parkinson disease. Neurology 67:1774–1780
Uc EY, Rizzo M, Anderson SW et al (2006) Impaired visual search in drivers with Parkinson’s disease. Ann Neurol 60:407–413
Uc EY, Rizzo M, Johnson AM et al (2009) Road safety in drivers with Parkinson disease. Neurology 73:2112–2119
Crizzle AM, Myers AM, Roy EA, Almeida QJ (2013) Drivers with Parkinson’s disease: are the symptoms of PD associated with restricted driving practices? J Neurol 260:2562–2568
Madeley P, Hulley JL, Wildgust H, Mindham RHS (1990) Parkinson’s disease and driving ability. J Neurol Neurosurg Psychiatry 53:580–582
Devos H, Vandenberghe W, Nieuwboer A et al (2007) Predictors of fitness to drive in people with Parkinson disease. Neurology 69:1434–1441
Zesiewicz TA, Cimino CR, Malek AR et al (2002) Driving safety in Parkinson’s disease. Neurology 59:1787–1788
Uc EY, Dastrup E (2009) Driving under low-contrast visibility conditions in Parkinson disease. Neurology 73:1103–1110
Scally K, Charlton JL, Iansek R et al (2011) Impact of external cue validity on driving performance in Parkinson’s disease. Parkinson’s Disease 2011:1–10
Stolwyk RJ, Triggs TJ, Charlton JL et al (2005) Impact of internal versus external cueing on driving performance in people with Parkinson’s disease. Mov Disord 20:846–857
Classen S, Holmes JD, Alvarez L et al (2015) Clinical assessments as predictors of primary on-road outcomes in Parkinson’s disease. OTJR 35:213–220
Ranchet M, Paire-ficout L, Marin-Lamellet C et al (2011) Impaired updating ability in drivers with Parkinson’s disease. J Neurol Neurosurg Psychiatry 82:218–224
Ranchet M, Paire-Ficout L, Uc EY et al (2013) Impact of specific executive functions on driving performance in people with Parkinson’s disease. Mov Disord 28:1941–1948
Worringham CJ, Wood JM, Kerr GK, Silburn PA (2006) Predictors of driving assessment outcome in Parkinson’s disease. Mov Disord 21:230–235
Ranchet M, Broussolle E, Paire-Ficout L (2016) Longitudinal executive changes in drivers with Parkinson’s disease: study using neuropsychological and driving simulator tasks. Eur J Neurol 76:143–150
Barrash J, Stillman A, Anderson SW et al (2010) Prediciton of driving ability with neuropsychological tests: demographic adjustments diminish accuracy. J Int Neuropsychol Soc 16:679–686
Barco PP, Baum CM, Ott BR et al (2015) Driving errors in persons with dementia. J Am Geriatr Soc 63:1373–1380
Bhalla RK, Papandonatos GD, Stern RA, Ott BR (2007) Anxiety of Alzheimer’s disease patients before and after a standardized on-road driving test. Alzheimer’s Dementia 3:33–39
Carr DB, Barco PP, Wallendorf MJ et al (2011) Predicting road test performance in drivers with dementia. J Am Geriatr Soc 59:2112–2117
Duchek JM, Carr DB, Hunt L et al (2003) Longitudinal driving performance in early-stage dementia of the Alzheimer type. J Am Geriatr Soc 51:1342–1347
Fox GK, Bowden SC, Bashford GM, Smith DS (1997) Alzheimer’s disease and driving: prediction and assessment of driving performance. J Am Geriatr Soc 45:949–953
Hunt LA, Murphy CF, Carr D et al (1997) Environmental cueing may affect performance on a road test for drivers with dementia of the Alzheimer type. Alzheimer Dis Assoc Disord 11:13–16
Lincoln NB, Radford KA, Lee E, Reay AC (2006) The assessment of fitness to drive in people with dementia. Int J Geriatr Psychiatry 21:1044–1051
Ott BR, Anthony D, Papandonatos GD et al (2005) Clinician assessment of the driving competence of patients with dementia. J Am Geriatr Soc 53:829–833
Ott BR, Festa EK, Amick MM et al (2008) Computerized maze navigation and on-road performance by drivers with dementia. J Geriatr Psychiatry Neurol 21:18–25
Ott BR, Heindel WC, Papandonatos GD et al (2008) A longitudinal study of drivers with Alzheimer disease. Neurology 70:1171–1178
Piersma D, Fuermaier ABM, De Waard D et al (2016) Prediction of fitness to drive in patients with Alzheimer’s dementia. PLoS One 11:1–29
Brown LB, Ott BR, Papandonatos GD et al (2005) Prediction of on-road driving performance in patients with early Alzheimer’s disease. J Am Geriatr Soc 53:94–98
Brown LB, Stern RA, Cahn-Weiner DA et al (2005) Driving scenes test of the neuropsychological assessment battery (NAB) and on-road driving performance in aging and very mild dementia. Arch Clin Neuropsychol 20:209–215
Dawson JD, Anderson SW, Uc EY et al (2009) Predictors of driving safety in early Alzheimer disease. Neurology 72:521–527
Fitten LJ, Perryman KM, Wilkinson CJ et al (1995) Alzheimer and vascular dementias and driving: a prospective road and laboratory study. JAMA 273:1360–1365
Manning KJ, Davis JD, Papandonatos GD, Ott BR (2014) Clock drawing as a screen for impaired driving in aging and dementia: is it worth the time? Arch Clin Neuropsychol 29:1–6
Uc EY, Rizzo M, Anderson SW et al (2004) Driver route-following and safety errors in early Alzheimer disease. Neurology 63:832–837
Uc EY, Rizzo M, Anderson SW et al (2005) Driver landmark and traffic sign identification in early Alzheimer’s disease. J Neurol Neurosurg Psychiatry 76:764–768
Duchek JM, Hunt L, Ball K et al (1998) Attention and driving performance in Alzheimer’s disease. J Gerontol 53:130–141
Aksan N, Anderson SW, Dawson J et al (2015) Cognitive functioning differentially predicts different dimensions of older drivers’ on-road safety. Accid Anal Prev 75:236–244
Grace J, Amick MM, D’Abreu A et al (2005) Neuropsychological deficits associated with driving performance in Parkinson’s and Alzheimer’s disease. J Int Neuropsychol Soc 11:766–775
Paire-Ficout L, Marin-Lamellet C, Lafont S et al (2016) The role of navigation instruction at intersections for older drivers and those with early Alzheimer’s disease. Accid Anal Prev 96:249–254
Uc EY, Rizzo M, Anderson SW et al (2006) Unsafe rear-end collision avoidance in Alzheimer’s disease. J Neurol Sci 251:35–43
Bieliauskas LA, Roper BR, Trobe J et al (1998) Cognitive measures, driving safety, and Alzheimer’s disease. Clin Neuropsychol 12:206–212
Cox DJ, Quillian WC, Thorndike FP et al (1998) Evaluating driving performance of outpatients with Alzheimer disease. J Am Board Family Pract 11:264–271
Frittelli C, Borghetti D, Iudice G et al (2009) Effects of Alzheimer’s disease and mild cognitive impairment on driving ability: a controlled clinical study by simulated driving test. Int J Geriatr Psychiatry 24:232–238
Stein AC, Dubinsky RM (2011) Driving simulator performance in patients with possible and probable Alzheimer’s disease. Ann Adv Automot Med 55:325–334
Yamin S, Stinchcombe A, Gagnon S (2016) Deficits in attention and visual processing but not global cognition predict simulated driving errors in drivers diagnosed with mild Alzheimer’s disease. Am J Alzheimer’s Dis Other Dementias 31:351–360
Rizzo M, McGehee DV, Dawson JD, Anderson SN (2001) Simulated car crashes at intersections in drivers with Alzheimer disease. Alzheimer Dis Assoc Disord 15:10–20
Rizzo M, Reinach S, Mcgehee D, Dawson J (1997) Simulated car crashes and crash predictors in drivers with Alzheimer disease. Arch Neurol 54:545–551
Yi J, Lee C, Parsons R, Falkmer T (2015) The effect of the global positioning system on the driving performance of people with mild Alzheimer’s disease. Gerontology 61:79–88
Lafont S, Marin-Lamellet C, Paire-Ficout L et al (2010) The Wechsler Digit Symbol Substitution Test as the best indicator of the risk of impaired driving in Alzheimer disease and normal aging. Dement Geriatr Cogn Disord 29:154–163
Bixby K, Davis JD, Ott BR (2015) Comparing varegiver and clinician predictions of fitness to drive in people with Alzheimer’s disease. Am J Occup Ther 69:1–7
Wild K, Cotrell V (2003) Identifying driving impairment in Alzheimer disease: a comparison of self and observer reports versus driving evaluation. Alzheimer Dis Assoc Disord 17:27–34
de Winter JCF, van Leeuwen PM, Happee R (2012) Advantages and disadvantages of driving simulators: a discussion. In: Proceedings of measuring behavior conference, pp 47–50
de Winter JCF, de Groot S, Mulder M et al (2009) Relationships between driving simulator performance and driving test results. Ergonomics 52:137–153
Mayhew DR, Simpson HM, Wood KM et al (2011) On-road and simulated driving: concurrent and discriminant validation. J Saf Res 42:267–275
Lee HC, Cameron D, Lee AH (2003) Assessing the driving performance of older adult drivers: on-road versus simulated driving. Accid Anal Prev 35:797–803
Devos H, Morgan JC, Onyeamaechi A et al (2016) Use of a driving simulator to improve on-road driving performance and cognition in persons with Parkinson’s disease: a pilot study. Aust Occup Ther J 63:408–414
Kennedy RS, Lane NE, Berbaum KS, Lilienthal MG (1993) Simulator Sickness Questionnaire: an enhanced method for quantifying simulator sickness. Int J Aviat Psychol 3:203–220
Brooks JO, Goodenough RR, Crisler MC et al (2010) Simulator sickness during driving simulation studies. Accid Anal Prev 42:788–796
Domeyer JE, Cassavaugh ND, Backs RW (2013) The use of adaptation to reduce simulator sickness in driving assessment and research. Accid Anal Prev 53:127–132
Cassavaugh ND, Domeyer JE, Backs RW (2011) Lessons learned regarding simulator sickness in older adult drivers. In: Universal access in human-computer interaction, pp 263–269
Classen S, Bewernitz M, Shechtman O (2011) Driving simulator sickness: an evidence-based review of the literature. Am J Occup Ther 65:179–188
Matas NA, Nettelbeck T, Burns NR (2015) Dropout during a driving simulator study: a survival analysis. J Saf Res 55:159–169
Mullen NW, Weaver B, Riendeau JA et al (2010) Driving performance and susceptibility to simulator sickness: are they related? Am J Occup Ther 64:288–295
Reason JT, Brand JJ (1975) Motion sickness. Academic, London
Mehanna R, Moore S, Hou JG et al (2014) Comparing clinical features of young onset, middle onset and late onset Parkinson’s disease. Parkinsonism Relat Disord 20:530–534
Kester MI, Scheltens P (2009) Dementia: the bare essentials. Pract Neurol 9:241–251
Janvin CC, Larsen JP, Salmon DP et al (2006) Cognitive profiles of individual patients with Parkinson’s disease and dementia: comparison with dementia with Lewy Bodies and Alzheimer’s disease. Mov Disord 21:337–342
Vonsattel JP, Myers RH, Stevens TJ et al (1985) Neuropathological classification of Huntington’s disease. J Neuropathol Exp Neurol 44:559–577
Dumas E, van den Bogaard SJ, Middelkoop HAM, Roos RAC (2013) A review of cognition in Huntington’s disease. Front Biosci (Schol Ed) 5:1–18
Bronnick K, Emre M, Lane R et al (2007) Profile of cognitive impairment in dementia associated with Parkinson’s disease compared with Alzheimer’s disease. J Neurol Neurosurg Psychiatry 78:1064–1069
Hart EP, Marinus J, Burgunder JM et al (2013) Better global and cognitive functioning in choreatic versus hypokinetic-rigid Huntington’s disease. Mov Disord 28:1142–1145
Jacobs M, Hart EP, van Zwet EW et al (2016) Progression of motor subtypes in Huntington’s disease: a 6-year follow-up study. J Neurol 263:2080–2085
Dubinsky RM, Gray C, Husted D et al (1991) Driving in Parkinson’s disease. Neurology 41:517–520
Uitti RJ (2009) Parkinson’s disease and issues related to driving. Parkinsonism Relat Disord 15:S122–S125
Crizzle AM, Myers AM, Almeida QJ (2012) Drivers with Parkinson’s disease: who participates in research studies? Parkinsonism Relat Disord 18:833–836
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
M. Jacobs and E.P. Hart report no disclosures. R.A.C. Roos is member of the advisory board of UniQure and reports grants from Gossweiler Foundation, CHDI Foundation, Inc., and from TEVA Pharmaceuticals. All grants are going to the LUMC institute.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Jacobs, M., Hart, E.P. & Roos, R.A.C. Driving with a neurodegenerative disorder: an overview of the current literature. J Neurol 264, 1678–1696 (2017). https://doi.org/10.1007/s00415-017-8489-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-017-8489-9