Abstract
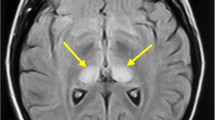
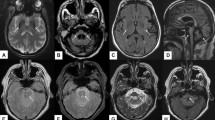
The objective of this study is to investigate the hyperintense lesions on diffusion-weighted magnetic resonance imaging (DWI) and its clinical correlation in sporadic Creutzfeldt–Jakob disease (sCJD). Patients who suffered from sCJD and followed up at the Department of Neurology at the General Hospital of the People’s Liberation Army during the period of June 1, 2007 to July 1, 2014 were reviewed. The location of the hyperintense lesions on DWI, apparent diffusion coefficient (ADC) values of the hyperintense lesions were correlated with symptoms and clinical course. A total of 58 sCJD patients and ten healthy controls were included. Hyperintense lesions on DWI were observed in all the patients. The patients with basal ganglia (BG) hyperintense lesions on DWI had shorter disease duration and higher incidence of myoclonus (92 versus 44 %) than those without BG hyperintense lesions. The patients with occipital cortex hyperintense lesions on DWI had shorter disease duration between symptom onset and akinetic mutism than those without these lesions. The lower of the BG ADC value the faster presence of akinetic mutism and the shorter disease duration the patients will have. The presence of BG and occipital cortex hyperintense lesions on DWI and BG ADC values is correlated with the clinical course and clinical symptoms.


Similar content being viewed by others
References
Lee J, Hyeon JW, Kim SY et al (2015) Review: laboratory diagnosis and surveillance of Creutzfeldt–Jakob disease. J Med Virol 87(1):175–186. doi:10.1002/jmv.24004
Geschwind MD, Potter CA, Sattavat M et al (2009) Correlating DWI MRI with pathological and other features of Jakob–Creutzfeldt disease. Alzheimer Dis Assoc Disord 23(1):82–87. doi:10.1097/WAD.0b013e31818323ef
Shiga Y, Miyazawa K, Sato S et al (2004) Diffusion-weighted MRI abnormalities as an early diagnostic marker for Creutzfeldt–Jakob disease. Neurology 63(3):443–449. doi:10.1212/01.WNL.0000134555.59460.5D
Demaerel P, Baert AL, Vanopdenbosch L et al (1997) Diffusion-weighted magnetic resonance imaging in Creutzfeldt–Jakob disease. Lancet 349(9055):847–848. doi:10.1016/S0140-6736(97)24012-2
Ukisu R, Kushihashi T, Kitanosono T et al (2005) Serial diffusion-weighted MRI of Creutzfeldt–Jakob disease. AJR Am J Roentgenol 184(2):560–566. doi:10.2214/ajr.184.2.01840560
Tschampa HJ, Kallenberg K, Urbach H et al (2005) MRI in the diagnosis of sporadic Creutzfeldt–Jakob disease: a study on inter-observer agreement. Brain 128(Pt 9):2026–2033. doi:10.1093/brain/awh575
Zhang J-T, Pu C-Q et al (2006) Consistency study of diffusion-weighted magnetic resonance imaging with clinical features and EEG in Creutzfeldt–Jakob disease. Chin J Neuromed 5(2):188–191. doi:10.3969/j.issn.1004-1648.2005.01.002
Hyare H, Thornton J, Stevens J et al (2010) High-b-value diffusion MR imaging and basal nuclei apparent diffusion coefficient measurements in variant and sporadic Creutzfeldt–Jakob disease. AJNR Am J Neuroradiol 31(3):521–526. doi:10.3174/ajnr.A1860
Kallenberg K, Schulz-Schaeffer WJ, Jastrow U et al (2006) Creutzfeldt–Jakob disease: comparative analysis of MR imaging sequences. AJNR Am J Neuroradiol 27(7):1459–1462
Murata T, Shiga Y, Higano S et al (2002) Conspicuity and evolution of lesions in Creutzfeldt–Jakob disease at diffusion-weighted imaging. AJNR Am J Neuroradiol 23(7):1164–1172
Young GS, Geschwind MD, Fischbein NJ et al (2005) Diffusion-weighted and fluid-attenuated inversion recovery imaging in Creutzfeldt–Jakob disease: high sensitivity and specificity for diagnosis. AJNR Am J Neuroradiol 26(6):1551–1562
Mittal S, Farmer P, Kalina P et al (2002) Correlation of diffusion-weighted magnetic resonance imaging with neuropathology in Creutzfeldt–Jakob disease. Arch Neurol 59(1):128–134. doi:10.1001/archneur.59.1.128
Matsusue E, Kinoshita T, Sugihara S et al (2004) White matter lesions in panencephalopathic type of Creutzfeldt–Jakob disease: MR imaging and pathologic correlations. AJNR Am J Neuroradiol 25(6):910–918
Meissner B, Kallenberg K, Sanchez-Juan P et al (2009) MRI lesion profiles in sporadic Creutzfeldt–Jakob disease. Neurology 72(23):1994–2001. doi:10.1212/WNL.0b013e3181a96e5d
Meissner B, Köhler K, Körtner K et al (2004) Sporadic Creutzfeldt–Jakob disease magnetic resonance imaging and clinical findings. Neurology 63(3):450–456. doi:10.1212/01.WNL.0000136225.80445.C9
Urbach H, Klisch J, Wolf HK et al (1998) MRI in sporadic Creutzfeldt–Jakob disease: correlation with clinical and neuropathological data. Neuroradiolog 40(2):65–70. doi:10.1007/s002340050542
Yi SH, Park KC, Yoon SS et al (2008) Relationship between clinical course and diffusion-weighted MRI findings in sporadic Creutzfeldt–Jakob disease. Neurol Sci 29(4):251–255. doi:10.1007/s10072-008-0976-4
Meissner B, Kallenberg K, Sanchez-Juan P et al (2008) Isolated cortical signal increase on MR imaging as a frequent lesion pattern in sporadic Creutzfeldt–Jakob disease. AJNR Am J Neuroradiol 29(8):1519–1524. doi:10.3174/ajnr.A1122
Kim JH, Choi BS, Jung C et al (2011) Diffusion-weighted imaging and magnetic resonance spectroscopy of sporadic Creutzfeldt–Jakob disease: correlation with clinical course. Neuroradiology 53(12):939–945. doi:10.1007/s00234-010-0820-4
Zerr I, Kallenberg K, Summers DM et al (2009) Updated clinical diagnostic criteria for sporadic Creutzfeldt–Jakob disease. Brain 132(Pt 10):2659–2668. doi:10.1093/brain/awp191
Tschampa HJ, Kallenberg K, Kretzschmar HA et al (2007) Pattern of cortical changes in sporadic Creutzfeldt–Jakob disease. AJNR Am J Neuroradiol 28(6):1114–1118. doi:10.3174/ajnr.A0496
Collie DA, Summers DM, Sellar RJ et al (2003) Diagnosing variant Creutzfeldt–Jakob disease with the pulvinar sign: MR imaging findings in 86 neuropathologically confirmed cases. AJNR Am J Neuroradiol 24(8):1560–1569
Lin YR, Young GS, Chen NK et al (2006) Creutzfeldt–Jakob disease involvement of rolandic cortex: a quantitative apparent diffusion coefficient evaluation. AJNR Am J Neuroradiol 27:1755–1759
Hakulinen U, Brander A, Ryymin P et al (2012) Repeatability and variation of region-of-interest methods using quantitative diffusion tensor MR imaging of the brain. BMC Med Imaging 12(1):30. doi:10.1186/1471-2342-12-30
Nogueira L, Brandão S, Matos E et al (2015) Region of interest demarcation for quantification of the apparent diffusion coefficient in breast lesions and its interobserver variability. Diagn Interv Radiol 21(2):123–127. doi:10.5152/dir.2014.14217
Kim JH, Lim MK, Jeon TY et al (2011) Diffusion and perfusion characteristics of melas (mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episode) in thirteen patients. Korean J Radiol 12(1):15–24. doi:10.3348/kjr.2011.12.1.15
Iwasaki Y, Mimuro M, Yoshida M et al (2011) Survival to akinetic mutism state in Japanese cases of MM1-type sporadic Creutzfeldt–Jakob disease is similar to Caucasians. Eur J Neurol 18(7):999–1002. doi:10.1111/j.1468-1331.2010.03185.x
Zerr I, Bodemer M, Gefeller O et al (1998) Detection of 14-3-3 protein in the cerebrospinal fluid supports the diagnosis of Creutzfeldt–Jakob disease. Ann Neurol 43(1):32–40. doi:10.1002/ana.410430109
Kretzschmar HA, Ironside JW, DeArmond SJ et al (1996) Diagnostic criteria for sporadic Creutzfeldt–Jakob disease. Arch Neurol 53(9):913–920. doi:10.1001/archneur.1996.00550090125018
Van Everbroeck B, Pals P, Dziedzic T, Dom R, Godfraind C et al (2000) Retrospective study of Creutzfeldt–Jakob disease in Belgium: neuropathological findings. Acta Neuropathol 99(4):358–364. doi:10.1007/s004010051136
Binelli S, Agazzi P, Canafoglia L et al (2010) Myoclonus in Creutzfeldt–Jakob disease: polygraphic and video-electroencephalography assessment of 109 patients. Mov Disord 25(16):2818–2827. doi:10.1002/mds.23397
Tschampa HJ, Mürtz P, Flacke S et al (2003) Thalamic involvement in sporadic Creutzfeldt–Jakob disease: a diffusion-weighted MR imaging study. AJNR Am J Neuroradiol 24(5):908–915
Rabinovici GD, Wang PN, Levin J et al (2006) First symptom in sporadic Creutzfeldt–Jakob disease. Neurology 66(2):286–287. doi:10.1212/01.wnl.0000196440.00297.67
Acknowledgments
The authors thank the Diagnostic Radiology Department and the Nuclear Medicine Department of the General Hospital of the People’s Liberation Army for their assistance.
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical standard
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding authors
Additional information
T. Gao and J.-H. Lyu contributed equally to the manuscript.
Rights and permissions
About this article
Cite this article
Gao, T., Lyu, JH., Zhang, JT. et al. Diffusion-weighted MRI findings and clinical correlations in sporadic Creutzfeldt–Jakob disease. J Neurol 262, 1440–1446 (2015). https://doi.org/10.1007/s00415-015-7723-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-015-7723-6