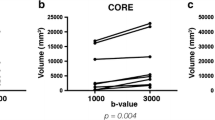
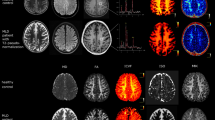
Abstract
Hereditary diffuse leukoencephalopathy with axonal spheroids (HDLS) is a rare autosomal dominant disease caused by mutations within the colony stimulating factor 1 receptor (CSF1R) gene. While a small number of reports on imaging findings in routine MRI exist, reported imaging findings in DWI and spectroscopy are scarce, and limited to not genetically proven case reports. We assessed MRI including DWI and MR spectroscopy in six patients with HDLS and two asymptomatic mutation carriers. A total of 13 MRIs were evaluated and a score of the white-matter lesion (WML) load was calculated. The course of MR abnormalities was followed for 6–19 months in four patients and 95 months in one carrier. MRI revealed widespread white-matter lesions of patchy or confluent pattern especially in the frontal and occipital lobe. The pyramidal tract was less affected than the surrounding tissue in all symptomatic patients on conventional T2WI. Three of four cases with DWI showed small dots of diffusion restriction within WML. Spectroscopy showed increased levels of mIns, Cho and lactate while NAA was decreased. Asymptomatic mutation carriers had, for the age of the patients, unusually pronounced unspecific WMLs. No diffusion restriction or alterations in metabolite levels could be detected in asymptomatic mutation carriers. Microbleeds were not found in any patient. Diffusion restriction seems to be a typical imaging pattern visible in patients with active disease progression in HDLS. Spectroscopic findings and the absence of microbleeds differ clearly from reported findings in CADASIL and subcortical arteriosclerotic encephalopathy. While the distribution and character of WMLs in asymptomatic cases remain unspecific they are likely to represent subclinical markers of HDLS.




Similar content being viewed by others
References
Axelsson R, Röyttä M, Sourander P, Akesson HO, Andersen O (1984) Hereditary diffuse leucoencephalopathy with spheroids. Acta Psychiatr Scand Suppl 314:1–65
Rademakers R, Baker M, Nicholson AM, Rutherford NJ, Finch N, Soto-Ortolaza A, Lash J, Wider C, Wojtas A, DeJesus-Hernandez M, Adamson J, Kouri N, Sundal C, Shuster EA, Aasly J, MacKenzie J, Roeber S, Kretzschmar HA, Boeve BF, Knopman DS, Petersen RC, Cairns NJ, Ghetti B, Spina S, Garbern J, Tselis AC, Uitti R, Das P, Jam Van Gerpen et al (2011) Mutations in the colony stimulating factor 1 receptor (CSF1R) gene cause hereditary diffuse leukoencephalopathy with spheroids. Nat Genet 44:200–205
Wider C, Van Gerpen JA, DeArmond S, Shuster EA, Dickson DW, Wzzolek ZK (2009) Leukoencephalopathy with spheroids (HDLS) and pigmentary leukodystrophy (POLD): a single entity? Neurology 72:1953–1959
Nicholson AM, Baker MC, Finch NA, Rutherford NJ, Wider C, Graff-Radford NR, Nelson PT, Clark HB, Wszolek ZK, Dickson DW, Knopman DS, Rademakers R (2013) CSF1R mutations link POLD and HDLS as a single disease entity. Neurology 80:1033–1040
Sundal C, Van Gerpen JA, Nicholson AM, Wider C, Shuster EA, Aasly J, Spina S, Ghetti B, Roeber S, Garbern J, Borjesson-Hanson A, Tselis A, Swerdlow RH, Miller BB, Fujioka S, Heckman MG, Uitti RJ, Josephs KA, Baker M, Andersen O, Rademakers R, Dickson DW, Broderick D, Wszolek ZK (2012) MRI characteristics and scoring in HDLS due to CSF1R gene mutations. Neurology 79:566–574
Keegan BM, Giannini C, Parisi JE, Lucchinetti CF, Boeve BF, Josephs KA (2008) Sporadic adult-onset leukoencephalopathy with neuroaxonal spheroids mimicking cerebral MS. Neurology 70:1128–1133
Itoh K, Shiga K, Shimizu K, Muranishi M, Nakagawa M, Fushiki S (2006) Autosomal dominant leukodystrophy with axonal spheroids and pigmented glia: clinical and neuropathological characteristics. Acta Neuropathol 111:39–45
Kinoshita M, Yoshida K, Oyanagi K, Hashimoto T, Ikeda S (2012) Hereditary diffuse leukoencephalopathy with axonal spheroids caused by R782H mutation in CSF1R: case report. J Neurol Sci 318:115–118
Kondo Y, Kinoshita M, Fukushima K, Yoshida K, Ikeda S (2013) Early involvement of the corpus callosum in a patient with hereditary diffuse leukoencephalopathy with spheroids carrying the de novo K793T mutation of CSF1R. Intern Med 52:503–506
Karle KN, Biskup S, Schüle R, Schweitzer KJ, Krüger R, Bauer P, Bender B, Nägele T, Schöls L (2013) De novo mutations in hereditary diffuse leukoencephalopathy with axonal spheroids (HDLS). Neurology 81:2039–2044
Hoffmann S, Murrell J, Harms L, Miller K, Meisel A, Brosch T, Scheel M, Ghetti B, Goebel HH, Stenzel W (2014) Enlarging the nosological spectrum of hereditary diffuse leukoencephalopathy with axonal spheroids (HDLS). Brain Pathol. doi:10.1111/bpa.12120 (in press)
Konno T, Tada M, Tada M, Koyama A, Nozaki H, Harigaya Y, Nishimiya J, Matsunaga A, Yoshikura N, Ishihara K, Arakawa M, Isami A, Okazaki K, Yokoo H, Itoh K, Yoneda M, Kawamura M, Inuzuka T, Takahashi H, Nishizawa M, Onodera O, Kakita A, Ikeuchi T (2014) Haploinsufficiency of CSF-1R and clinicopathologic characterization in patients with HDLS. Neurology 82:139–148
Terasawa Y, Osaki Y, Kawarai T, Sugimoto T, Orlacchio A, Abe T, Izumi Y, Kaji R (2013) Increasing and persistent DWI changes in a patient with hereditary diffuse leukoencephalopathy with spheroids. J Neurol Sci 335:213–214
Mateen FJ, Keegan BM, Krecke K, Parisi JE, Trenerry MR, Pittock SJ (2010) Sporadic leucodystrophy with neuroaxonal spheroids: persistence of DWI changes and neurocognitive profiles: a case study. J Neurol Neurosurg Psychiatry 81:619–622
Boissé L, Islam O, Woulfe J, Ludwin SK, Brunet DG (2010) Hereditary diffuse leukoencephalopathy with neuroaxonal spheroids: novel imaging findings. J Neurol Neurosurg Psychiatry 81:313–340
Sundal C, Ekholm S, Nordborg C, Jönsson L, Börjesson-Hanson A, Lindén T, Zetterberg H, Viitanen M, Andersen O (2012) Update of the original HDLS kindred: divergent clinical causes. Acta Neurol Scand 126:67–75
Freeman SH, Hyman BT, Sims KB, Hedley-Whyte ET, Vossough A, Frosch MP, Schmahmann JD (2009) Adult onset leukodystrophy with neuroaxonal spheroids: clinical, neuroimaging and neuropathologic observations. Brain Pathol 19:39–47
Sundal C, Lash J, Aasly J, Øygarden S, Roeber S, Kretzschman H, Garbern JY, Tselis A, Rademakers R, Dickson DW, Broderick D, Wszolek ZK (2012) Hereditary diffuse leukoencephalopathy with axonal spheroids (HDLS): a misdiagnosed disease ententity. J Neurol Sci 314:130–137
Sohn SY, Ko YJ, Hong JM, Kim SH, Kim HS, Kim JH, Chi JG, Moon SY (2010) A case of pigmentary orthochromatic leukodystrophy with findings of proton MR spectroscopy and serial brain MRIs. J Neurol Sci 295:23–26
Terada S, Ishizu H, Yokota O, Ishihara T, Nakashima H, Kugo A, Tanaka Y, Nakashima T, Nakashima Y, Kuroda S (2004) An autopsy case of hereditary diffuse leukoencephalopathy with spheroids, clinically suspected of Alzheimer’s disease. Acta Neuropathol 108:538–545
Mendes A, Pinto M, Vieira S, Castro L, Carpenter S (2010) Adult-onset leukodystrophy with axonal spheroids. J Neurol Sci 297:40–45
Baba Y, Ghetti B, Baker MC, Uitti RJ, Hutton ML, Yamaguchi K, Bird T, Lin W, DeLucia MW, Dickson DW, Wszolek ZK (2006) Hereditary diffuse leukoencephalopathy with spheroids: clinical, pathologic and genetic studies of a new kindred. Acta Neuropathol 111:300–311
Yamashita M, Yamamoto T (2002) Neuroaxonal leukencephalopathy with axonal spheroids. Eur Neurol 48:20–25
Browne L, Sweeney BJ, Farrell MA (2003) Late-onset neuroaxonal leukoencephalopathy with spheroids and vascular amyloid. Eur Neurol 50:85–90
Van Gerpen JA, Wider C, Broderick DF, Dickson DW, Brown LA, Wszolek ZK (2008) Insights into the dynamics of hereditary diffuse leukoencephalopathy with axonal spheroids. Neurology 71:925–929
Sundal C, Ekholm S, Andersen O (2010) White matter disorders with autosomal dominant heredity: a review with personal clinical case studies and there MRI findings. Acta Neurol Scand 121:328–337
Mayer B, Oelschlaeger C, Keyvani K, Niederstadt T (2007) Two cases of LENAS: diagnosis by MRI and biopsy. J Neurol 254:1453–1454
Maillart E, Rousseau A, Galanaud D, Gray F, Polivka M, Labauge P, Hauw JJ, Lyon-Caen O, Gout O, Sedel F (2009) Rapid onset frontal leukodystrophy with decreased diffusion coefficient and neuroaxonal spheroids. J Neurol 256:1649–1654
Moro-de-Casillas ML, Cohen ML, Riley DE (2004) Leukoencephalopathy with neuroaxonal spheroids (LENAS) presenting as the cerebellar subtype of multiple system atrophy. J Neurol Neurosurg Psychiatry 75:1070–1072
Hancock N, Poon M, Taylor B, McLean C (2003) Hereditary diffuse leukencephalopathy with spheroids. J Neurol Neurosurg Psychiatry 74:1345–1347
van der Knaap MS, Naidu S, Kleinschmidt-Demasters BK, Kamphorst W, Weinstein HC (2000) Autosomal dominant diffuse leukoencephalopathy with neuroaxonal spheroids. Neurology 54:463–479
Glanville NT, Byers DM, Cook HW, Spence MW, Palmer FB (1989) Differences in the metabolism of inositol and phosphoinositides by cultured cells of neuronal and glial origin. Biochim Biophys Acta 1004:169–179
Ross BD, Bluml S, Cowan R, Danielsen E, Farrow N, Gruetter R (1997) In vivo magnetic resonance spectroscopy of human brain: the biophysical basis of dementia. Biophys Chem 68:161–172
Ross B, Bluml S (2001) Magnetic resonance spectroscopy of the human brain. Anat Rec 265:54–84
López-Villegas D, Lenkinski RE, Wehrli SL, Douglas SD (1995) Lactate production by human monocytes/macrophages determined by proton MR spectroscopy. Magn Reson Med 34:32–38
Bender B, Bornemann A, Reimold M, Ernemann U, Horger M (2012) Imaging findings in autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL)—CADASIL—the most frequent familial stroke syndrome. Rofo 184:679–683
Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG (2009) Cadasil. Lancet Neurol 8:643–653
Akhvlediani T, Henning A, Sándor PS, Boesiger P, Jung HH (2010) Adaptive metabolic changes in CADASIL white matter. J Neurol 257:171–177
Yao M, Jouvent E, Durin M, Godin O, Hervé D, Guichard JP, Zhu YC, Gschwendtner A, Opherk C, Dichgans M, Chabriat H (2012) Extensive white matter hyperintensities may increase brain volume in cerebral autosomal-dominant arteriopathy with subcortical infarct and leukoencephalopathy. Stroke 43:3252–3257
Kinoshita M, Kondo Y, Yoshida K, Fukushima K, Hoshi K, Ishizawa K, Araki N, Yazawa I, Washimi Y, Saitoh B, Kira J, Ikeda S (2014) Corpus callosum atrophy in patients with hereditary diffuse leukoencephalopathy with neuroaxonal spheroids: an MRI-based study. Intern Med 53:21–27
Kantarci K, Petersen C, Boeve BF, Knopman DS, Tang-Wai DF, O’Brien PC, Weigand SD, Edland SD, Smith GE, Ivnik RJ, Ferman TJ, Tangalos EG, Jack CR Jr (2004) 1H MR spectroscopy in common dementias. Neurology 63:1393–1398
Nitkunan A, Charlton RA, Barrick TR, McIntyre DJO, Howe FA, Markus HS (2009) Reduced N-aceylaspartate is consistent with axonal dysfunction in cerebral small vessel disease. NMR Biomed 22:285–291
Seo SW, Lee BH, Kim E-J, Chin J, Sun Cho Y, Yoon U, Na DL (2007) Clinical significance of microbleeds in subcortical vascular dementia. Stroke 38:1949–1951
Balashov KE, Aung LL, Dhib-Jalbut S, Keller IA (2011) Acute multiple sclerosis lesion: conversion of restricted diffusion due to vasogenic edema. J Neuroimaging 21:202–204
Christiansen P, Larsson HB, Thomsen C, Wieslander SB, Henriksen O (1994) Age dependent white matter lesions and brain volume changes in healthy volunteers. Acta Radiol 35:117–122
Acknowledgments
The authors wish to thank Chris Nawrat for editing the manuscript for British grammar and punctuation.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical standard
The study was approved by the local institutional review board and written informed consent was obtained from all patients.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
415_2014_7509_MOESM1_ESM.tif
Online resource 1: Increased lesion load and progressive atrophy in HDLS after 14 months. Two sagital T2-weighted slices of the same patient (Nr. 6) with a time interval of 14 months (initial scan left side, control scan right side). There is a progressive atrophy pronounced in the corpus callosum, less prominent in the frontal lobe. The patchy areas of hyperintense signal in the frontal and parietal lobe are more confluent in the follow-up scan (TIFF 3130 kb)
415_2014_7509_MOESM2_ESM.tif
Online resource 2: Typical lesion distribution in HDLS and CADASIL. A typical case of HDLS (bottom row) in comparison to a typical case of CADASIL (top row). WML are not found within the temporal lobe and the region of the basal ganglia in this case of HDLS, typical involvement of the capsula externa and the temporal pole is seen in CADASIL. A mild atrophy of the frontal lobe is present in HDLS but no atrophy is seen in CADASIL (TIFF 2782 kb)
Rights and permissions
About this article
Cite this article
Bender, B., Klose, U., Lindig, T. et al. Imaging features in conventional MRI, spectroscopy and diffusion weighted images of hereditary diffuse leukoencephalopathy with axonal spheroids (HDLS). J Neurol 261, 2351–2359 (2014). https://doi.org/10.1007/s00415-014-7509-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-014-7509-2