Abstract
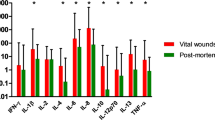
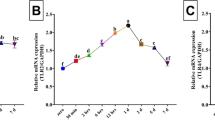
Interleukin (IL)-33, an important inflammatory cytokine, is highly expressed in skin wound tissue and serum of humans and mice, and plays an essential role in the process of skin wound healing (SWH) dependent on the IL-33/suppression of tumorigenicity 2 (ST2) pathway. However, whether IL-33 and ST2 themselves, as well as their interaction, can be applied for skin wound age determination in forensic practice remains incompletely characterized. Human skin samples with injured intervals of a few minutes to 24 hours (hs) and mouse skin samples with injured intervals of 1 h to 14 days (ds) were collected. Herein, the results demonstrated that IL-33 and ST2 are increased in the human skin wounds, and that in mice skin wounds, there is an increase over time, with IL-33 expression peaking at 24 hs and 10 ds, and ST2 expression peaking at 12 hs and 7 ds. Notably, the relative quantity of IL-33 and ST2 proteins < 0.35 suggested a wound age of 3 hs; their relative quantity > 1.0 suggested a wound age of 24 hs post-mouse skin wounds. In addition, immunofluorescent staining results showed that IL-33 and ST2 were consistently expressed in the cytoplasm of F4/80-positive macrophages and CD31-positive vascular endothelial cells with or without skin wounds, whereas nuclear localization of IL-33 was absent in α-SMA-positive myofibroblasts with skin wounds. Interestingly, IL-33 administration facilitated the wound area closure by increasing the proliferation of cytokeratin (K) 14 -positive keratinocytes and vimentin-positive fibroblasts. In contrast, treating with its antagonist (i.e., anti-IL-33) or receptor antagonist (e.g., anti-ST2) exacerbated the aforementioned pathological changes. Moreover, treatment with IL-33 combined with anti-IL-33 or anti-ST2 reversed the effect of IL-33 on facilitating skin wound closure, suggesting that IL-33 administration facilitated skin wound closure through the IL-33/ST2 signaling pathway. Collectively, these findings indicate that the detection of IL-33/ST2 might be a reliable biomarker for the determination of skin wound age in forensic practice.




Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in Figs. 1–5. Additional data sets that support the findings of this study are available from the corresponding author upon reasonable request.
References
Kim TH, Jeon WY, Ji Y et al (2021) Electricity auto-generating skin patch promotes wound healing process by activation of mechanosensitive ion channels. Biomaterials 275:120948. https://doi.org/10.1016/j.biomaterials.2021.120948
Zhu W, Zhai X, Jia Z, Wang Y, Mo Y (2022) Bioinformatics analysis of sequential gene expression profiling after skin and skeletal muscle wound in mice. Leg Med (Tokyo) 54:101982. https://doi.org/10.1016/j.legalmed.2021.101982
Peyron PA, Colomb S, Becas D et al (2021) Cytokines as new biomarkers of skin wound vitality. Int J Legal Med 135:2537–2545. https://doi.org/10.1007/s00414-021-02659-z
Kuninaka Y, Ishida Y, Nosaka M et al (2020) Forensic pathological study on temporal appearance of dendritic cells in skin wounds. Int J Legal Med 134:597–601. https://doi.org/10.1007/s00414-019-02185-z
Grellner W (2002) Time-dependent immunohistochemical detection of proinflammatory cytokines (IL-1beta, IL-6, TNF-alpha) in human skin wounds. Forensic Sci Int 130:90–96. https://doi.org/10.1016/s0379-0738(02)00342-0
Dewan MC, Rattani A, Gupta S et al (2018) Estimating the global incidence of traumatic brain injury. J Neurosurg 130(4):1080–1097. https://doi.org/10.3171/2017.10.JNS17352
Niedecker A, Huhn R, Ritz-Timme S, Mayer F (2021) Complex challenges of estimating the age and vitality of muscle wounds: a study with matrix metalloproteinases and their inhibitors on animal and human tissue samples. Int J Legal Med 135:1843–1853. https://doi.org/10.1007/s00414-021-02563-6
Birincioğlu İ, Akbaba M, Alver A et al (2016) Determination of skin wound age by using cytokines as potential markers. J Forensic Leg Med 44:14–19. https://doi.org/10.1016/j.jflm.2016.08.011
Kondo T, Ohshima T (1996) The dynamics of inflammatory cytokines in the healing process of mouse skin wound: a preliminary study for possible wound age determination. Int J Legal Med 108:231–236. https://doi.org/10.1007/BF01369816
Wang Y, Yamamoto Y, Kuninaka Y, Kondo T, Furukawa F (2015) Forensic Potential of MMPs and CC Chemokines for Wound Age Determination. J Forensic Sci 60:1511–1515. https://doi.org/10.1111/1556-4029.12831
Lefrancais E, Roga S, Gautier V et al (2012) IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc Natl Acad Sci USA 109:1673–1678. https://doi.org/10.1073/pnas.1115884109
Schmitz J, Owyang A, Oldham E et al (2005) IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23:479–490. https://doi.org/10.1016/j.immuni.2005.09.015
Perez F, Ruera CN, Miculan E et al (2020) IL-33 Alarmin and Its Active Proinflammatory Fragments Are Released in Small Intestine in Celiac Disease. Front Immunol 11:581445. https://doi.org/10.3389/fimmu.2020.581445
Imai Y, Yasuda K, Sakaguchi Y et al (2013) Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice. Proc Natl Acad Sci USA 110:13921–13926. https://doi.org/10.1073/pnas.1307321110
Taniguchi S, Elhance A, Van Duzer A, Kumar S, Leitenberger JJ, Oshimori N (2020) Tumor-initiating cells establish an IL-33-TGF-β niche signaling loop to promote cancer progression. Science 369(6501):eaay1813. https://doi.org/10.1126/science.aay1813
Oshio T, Komine M, Tsuda H et al (2017) Nuclear expression of IL-33 in epidermal keratinocytes promotes wound healing in mice. J Dermatol Sci 85:106–114. https://doi.org/10.1016/j.jdermsci.2016.10.008
Liew FY (2012) IL-33: a Janus cytokine. Ann Rheum Dis 71(Suppl 2):i101–i104. https://doi.org/10.1136/annrheumdis-2011-200589
Komi DEA, Khomtchouk K, Santa Maria PL (2020) A Review of the Contribution of Mast Cells in Wound Healing: Involved Molecular and Cellular Mechanisms. Clin Rev Allergy Immunol 58:298–312. https://doi.org/10.1007/s12016-019-08729-w
Cannavo SP, Bertino L, Di Salvo E, Papaianni V, Ventura-Spagnolo E, Gangemi S (2019) Possible Roles of IL-33 in the Innate-Adaptive Immune Crosstalk of Psoriasis Pathogenesis. Mediators Inflamm 2019:7158014. https://doi.org/10.1155/2019/7158014
Dagher R, Copenhaver AM, Besnard V et al (2020) IL-33-ST2 axis regulates myeloid cell differentiation and activation enabling effective club cell regeneration. Nat Commun 11:4786. https://doi.org/10.1038/s41467-020-18466-w
Gao Y, Luo C, Rui T et al (2021) Autophagy inhibition facilitates wound closure partially dependent on the YAP/IL-33 signaling in a mouse model of skin wound healing. Faseb J 35:e21920. https://doi.org/10.1096/fj.202002623RRR
Gao Y, Zhang MY, Wang T et al (2018) IL-33/ST2L Signaling Provides Neuroprotection Through Inhibiting Autophagy, Endoplasmic Reticulum Stress, and Apoptosis in a Mouse Model of Traumatic Brain Injury. Front Cell Neurosci 12:95. https://doi.org/10.3389/fncel.2018.00095
Li X, Liu C, Zhu Y et al (2021) SETD2 epidermal deficiency promotes cutaneous wound healing via activation of AKT/mTOR Signalling. Cell Prolif 54:e13045. https://doi.org/10.1111/cpr.13045
Kondo A, Shahpasand K, Mannix R et al (2015) Antibody against early driver of neurodegeneration cis P-tau blocks brain injury and tauopathy. Nature 523:431–436. https://doi.org/10.1038/nature14658
Gao Y, Wang T, Cheng Y et al (2023) Melatonin ameliorates neurological deficits through MT2/IL-33/ferritin H signaling-mediated inhibition of neuroinflammation and ferroptosis after traumatic brain injury. Free Radic Biol Med 199:97–112. https://doi.org/10.1016/j.freeradbiomed.2023.02.014
Cheng Y, Gao Y, Li J et al (2023) TrkB agonist N-acetyl serotonin promotes functional recovery after traumatic brain injury by suppressing ferroptosis via the PI3K/Akt/Nrf2/Ferritin H pathway. Free Radic Biol Med 194:184–198. https://doi.org/10.1016/j.freeradbiomed.2022.12.002
Ma WX, Yu TS, Fan YY et al (2011) Time-dependent expression and distribution of monoacylglycerol lipase during the skin-incised wound healing in mice. Int J Legal Med 125:549–558. https://doi.org/10.1007/s00414-011-0567-4
Ishida Y, Kimura A, Takayasu T, Eisenmenger W, Kondo T (2008) Expression of oxygen-regulated protein 150 (ORP150) in skin wound healing and its application for wound age determination. Int J Legal Med 122:409–414. https://doi.org/10.1007/s00414-008-0255-1
Gu S, Dai H, Zhao X, Gui C, Gui J (2020) AKT3 deficiency in M2 macrophages impairs cutaneous wound healing by disrupting tissue remodeling. Aging (Albany NY) 12:6928–6946. https://doi.org/10.18632/aging.103051
Foster DS, Januszyk M, Yost KE et al (2021) Integrated spatial multiomics reveals fibroblast fate during tissue repair. Proc Natl Acad Sci USA 118:e2110025118. https://doi.org/10.1073/pnas.2110025118
Shpichka A, Butnaru D, Bezrukov EA et al (2019) Skin tissue regeneration for burn injury. Stem Cell Res Ther 10:94. https://doi.org/10.1186/s13287-019-1203-3
Yu Q, Dai Q, Huang Z et al (2023) Microfat exerts an anti-fibrotic effect on human hypertrophic scar via fetuin-A/ETV4 axis. J Transl Med 21:231. https://doi.org/10.1186/s12967-023-04065-y
Wang Y, Feng Q, Li Z, Bai X, Wu Y, Liu Y (2020) Evaluating the Effect of Integra Seeded with Adipose Tissue-Derived Stem Cells or Fibroblasts in Wound Healing. Curr Drug Deliv 17:629–635. https://doi.org/10.2174/1567201817666200512104004
Liew FY, Girard JP, Turnquist HR (2016) Interleukin-33 in health and disease. Nat Rev Immunol 16:676–689. https://doi.org/10.1038/nri.2016.95
Toki S, Goleniewska K, Zhang J et al (2020) TSLP and IL-33 reciprocally promote each other's lung protein expression and ILC2 receptor expression to enhance innate type-2 airway inflammation. Allergy 75:1606–1617. https://doi.org/10.1111/all.14196
Drake LY, Squillace D, Iijima K et al (2019) Early Life Represents a Vulnerable Time Window for IL-33-Induced Peripheral Lung Pathology. J Immunol 203:1952–1960. https://doi.org/10.4049/jimmunol.1900454
Du L, Hu X, Yang W et al (2019) Spinal IL-33/ST2 signaling mediates chronic itch in mice through the astrocytic JAK2-STAT3 cascade. Glia 67:1680–1693. https://doi.org/10.1002/glia.23639
Wulff BC, Pappa NK, Wilgus TA (2019) Interleukin-33 encourages scar formation in murine fetal skin wounds. Wound Repair Regen 27:19–28. https://doi.org/10.1111/wrr.12687
Rak GD, Osborne LC, Siracusa MC et al (2016) IL-33-Dependent Group 2 Innate Lymphoid Cells Promote Cutaneous Wound Healing. J Invest Dermatol 136:487–496. https://doi.org/10.1038/jid.2015.406
Pi L, Fang B, Meng X, Qian L (2022) LncRNA XIST accelerates burn wound healing by promoting M2 macrophage polarization through targeting IL-33 via miR-19b. Cell Death Discov 8:220. https://doi.org/10.1038/s41420-022-00990-x
He R, Yin H, Yuan B et al (2017) IL-33 improves wound healing through enhanced M2 macrophage polarization in diabetic mice. Mol Immunol 90:42–49. https://doi.org/10.1016/j.molimm.2017.06.249
Yin H, Li X, Hu S et al (2013) IL-33 accelerates cutaneous wound healing involved in upregulation of alternatively activated macrophages. Mol Immunol 56:347–353. https://doi.org/10.1016/j.molimm.2013.05.225
Yin H, Li X, Hu S et al (2013) IL-33 promotes Staphylococcus aureus-infected wound healing in mice. Int Immunopharmacol 17:432–438. https://doi.org/10.1016/j.intimp.2013.07.008
Xiao Y, Peng J, Liu Q et al (2020) Ultrasmall CuS@BSA nanoparticles with mild photothermal conversion synergistically induce MSCs-differentiated fibroblast and improve skin regeneration. Theranostics 10:1500–1513. https://doi.org/10.7150/thno.39471
Crompton RA, Williams H, Campbell L et al (2022) An Epidermal-Specific Role for Arginase1 during Cutaneous Wound Repair. J Invest Dermatol 142:1206–16.e8. https://doi.org/10.1016/j.jid.2021.09.009
Lee J, Jang H, Park S et al (2019) Platelet-rich plasma activates AKT signaling to promote wound healing in a mouse model of radiation-induced skin injury. J Transl Med 17:295. https://doi.org/10.1186/s12967-019-2044-7
Gurtner GC, Werner S, Barrandon Y, Longaker MT (2008) Wound repair and regeneration. Nature 453:314–321. https://doi.org/10.1038/nature07039
Li H, Han X, Zuo K et al (2018) miR-23b promotes cutaneous wound healing through inhibition of the inflammatory responses by targeting ASK1. Acta Biochim Biophys Sin (Shanghai) 50:1104–1113. https://doi.org/10.1093/abbs/gmy109
Altara R, Ghali R, Mallat Z, Cataliotti A, Booz GW, Zouein FA (2018) Conflicting vascular and metabolic impact of the IL-33/sST2 axis. Cardiovasc Res 114:1578–1594. https://doi.org/10.1093/cvr/cvy166
Küchler AM, Pollheimer J, Balogh J et al (2008) Nuclear interleukin-33 is generally expressed in resting endothelium but rapidly lost upon angiogenic or proinflammatory activation. Am J Pathol 173:1229–1242. https://doi.org/10.2353/ajpath.2008.080014
Cayrol C, Girard JP (2009) The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci USA 106:9021–9026. https://doi.org/10.1073/pnas.0812690106
Betz P, Nerlich A, Wilske J, Tubel J, Penning R, Eisenmenger W (1992) Time-dependent appearance of myofibroblasts in granulation tissue of human skin wounds. Int J Legal Med 105:99–103. https://doi.org/10.1007/BF02340832
Funding
This work was supported by the National Natural Science Foundation of China (82101972, 81530062, 81971800, 81971163, 82001382, and 82271409), Jiangsu Provincial Natural Science Foundation of China (SBK2020040785), Suzhou Municipal Science and Technology Bureau (SYS2019027), China Postdoctoral Science Foundation (2020M681723), Undergraduate Training Program for Innovation and Entrepreneurship, Soochow University (202110285044Z), a project of invigorating healthcare through science, technology, and education (KJXW2019018), and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Contributions
Yuan Gao, Luwei Cai, Ziguang Lei, and Luwen Zhu established the SWH model, and carried out molecular biology experiments; Yuan Gao, Chengliang Luo, and Luyang Tao analyzed the data and wrote the manuscript. Tao Wang, Dongya Li, Youzhuang Wu, and Hen Xu helped collect tissue samples. Yulu Wu, Wenjing Ren, Yirui Song, Dongya Li, and Lili Li participated in data interpretation. Yuan Gao and Chengliang Luo reviewed and edited the manuscript. All authors have contributed significantly to the design of this experiment, participated in the drafting and rigorous review of this manuscript, and endorsed its final version.
Corresponding authors
Ethics declarations
Research involving human participants and/or animals
The skin wound tissues of humans and mice involved in this study were obtained with the approval of the Ethics Committee of Soochow University and the Forensic Center of Soochow University.
Informed consent
Informed consent was obtained from all individual participants included in the study and/ or from legal representatives.
Ethical Approval
Ethical approval has been obtained from the Ethics Committee of Soochow University for animal experimentation and human Samples.
Conflicts of interest
No potential conflict of interest was reported by the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuan Gao, Luwei Cai and Dongya Li contributed equally to this work.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, Y., Cai, L., Li, D. et al. Extended characterization of IL-33/ST2 as a predictor for wound age determination in skin wound tissue samples of humans and mice. Int J Legal Med 137, 1287–1299 (2023). https://doi.org/10.1007/s00414-023-03025-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-023-03025-x