Abstract
Introduction
Bronchiolitis obliterans syndrome (BOS) is the most common form of CLAD and is characterized by airflow limitation and an obstructive spirometry pattern without parenchymal opacities. The protein signature of BOS lesions concerns extracellular matrix organization and aberrant basement membrane composition. In this pilot study, we investigated the presence of COL4A5 in the serum of patients with BOS.
Methods
41 patients who had undergone LTX were enrolled. Of these, 27 developed BOS and 14 (control group) were considered stable at the time of serum sampling. Of BOS patients, serum samples were analysed at the time of BOS diagnosis and before the clinical diagnosis (pre-BOS). COL4A5 levels were detected through the ELISA kit.
Results
Serum concentrations of COL4A5 were higher in pre-BOS than in stable patients (40.5 ± 13.9 and 24.8 ± 11.4, respectively, p = 0.048). This protein is not influenced by comorbidities, such as acute rejection or infections, or by therapies. Survival analysis also reveals that a higher level of COL4A5 was also associated with less probability of survival. Our data showed a correlation between concentrations of COL4A5 and FEV1 at the time of diagnosis of BOS.
Conclusion
Serum concentrations of COL4A5 can be considered a good prognostic marker due to their association with survival and correlation with functional parameters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung transplant (LTX) is a life-saving treatment option for patients with severe chronic lung diseases [1]. Around 50% of transplanted patients develop chronic lung allograft dysfunction (CLAD) within 5 years [2]. Bronchiolitis obliterans syndrome (BOS) is the most common form of CLAD and is characterized by airflow limitation and an obstructive spirometric pattern without high-resolution computed tomography (HRCT) evidence of parenchymal opacities [3, 4]. In particular, computed tomography (CT) and microCT analysis show abundant small-airway obstruction, starting from the fifth generation of airway branching and affecting up to 40–70% of airways [5]. Histological analysis shows severe mononuclear infiltration of the airways, vessels, and septum, associated with fibrosis [6, 7].
The pathogenesis of BOS remains unclear. It is a multifactorial syndrome that leads to pathological tissue changes and is associated with the worst long-term survival in LTX patients [8, 9]. For all these reasons, there have been attempts to identify markers for early diagnosis of BOS [10, 11].
The main trigger of BOS is a fibro-proliferative process called the epithelial–mesenchymal transition, derived from self-perpetuating activation of fibroblasts and massive release of collagen leading to peribronchiolar fibrosis and a decline in lung function [12, 13].
The protein signature of BOS lesions concerns extracellular matrix organization, wound healing, and aberrant basement membrane composition. Basement membrane components, such as epitopes on collagens and laminins, are targeted by auto-antibodies and lymphocytes in bronchiolitis obliterans syndrome arising after lung transplant [13, 14].
The main protein involved in the alteration of basement membrane structure is collagen [15]. Thickening of the lamina reticularis impairs airway distensibility and patency [16]. Collagen types IV and VI are two proteins abundant in basement membrane; their loss of integrity causes abnormal lung architecture followed by fibrosis [13]. Collagen types I and V are two collagens of the lamina reticularis, a thin layer below the basement membrane [17, 18]. They are network-forming collagens that assemble into sheet-like networks and underlie the epithelial and endothelial cells [19, 20]. Mature collagen IV is a heterotrimer comprised of a combination of two or three of the six known collagen IV alpha chains which are increasingly recognized as being tissue- and organ-specific [16, 21]. Silencing of COL4 alpha 5 chain (COL4A5) is reported to impair endothelial cell barrier integrity and to alter tight junction formation [22]. In this pilot study, we investigated the presence of COL4A5 in serum of patients with BOS. The protein was detected and could be a useful predictive marker of BOS.
Materials and Methods
Study Design and Population
In this single-centre retrospective study, we enrolled 41 patients who had undergone LTX at the Lung Transplant Centre of Siena University Hospital between 2012 and 2022 and from whom serum samples were available. Of these, 27 developed BOS and 14 (control group) were considered stable at the time of serum sampling.
For all patients, demographic data, age of transplant, type of transplant, and the pathologies that made transplant necessary were recorded. For BOS patients, days of CLAD-free survival, BAL cell count, and lymphocyte subsets were recorded together with therapy at onset of BOS, previous episodes of acute rejection, and infections. BOS was graded following ISHLT guidelines [23].
Lung function tests (LFT), including FEV1, FVC, and DLCO percentages, as well as serum samples from the BOS group were obtained at and 3–6 months before diagnosis of BOS and also at the moment of BOS diagnosis[24]. At the time of serum sampling, patients were negative for infection or acute rejection. Patients with concurrent infections at the time of serum samples were excluded from the study.
COL4A5 Detection
Serum concentrations of COL4A5 were measured by commercially available enzyme-linked-immuno-sorbent assay (ELISA kit, MyBioSource Inc) following the manufacturer’s instructions. Concentrations were read at 450 nm with a Victor X4 fluorimeter (Perkin Elmer Inc). Concentrations of COL4A5 were expressed in nanograms per millilitre (pg/ml).
Statistical Analysis
Means and standard deviations (M ± SD) or medians and quartiles (25th and 75th percentiles) for continuous variables were used. A non-parametric one-way ANOVA test (Kruskal–Wallis test) and Dunn test were performed for comparisons of more than two groups. The Shapiro–Wilk test was used to test normal distribution of the variables. The Chi-squared test was used for categorical variables. Receiver-operating characteristic (ROC) curve analysis with areas under curves (AUC) was performed to detect the best cut-off values for sensitivity and specificity. The Youden index [J = max (sensitivity + specificity − 1)] was used to establish the best cut-offs for diagnosis. The Spearman test was used to look for correlations among variables. We also performed binomial and multinomial logistic regression to identify the variable that most influenced serum concentrations of COL4A5 and airway restriction severity. Kaplan-Meyer analysis was performed to associate serum concentrations of COL4A5 with overall survival of the entire cohort and CLAD-free days of BOS patients. Statistical analysis and graphic representation of data were performed by GraphPad Prism 9.0 software (GraphPad Holdings, LLC, San Diego, CA, USA) and Jamovi Free software. A p value less than 0.05 was considered statistically significant.
Results
Cohort Description
The main clinical characteristics are reported in Table 1. No differences in terms of age and type of transplant were found (p > 0.05). A predominance of males emerged in the BOS group. COPD/Emphysema and IPF were the two diseases most frequent in our cohort before transplant. Regarding outcome, 32.4% of BOS patients died, whereas members of the stable group were all alive on 31 December 2022. In the BOS group, the mean number of CLAD-free days was 1073 ± 1007.32, with a mean of survival after transplant of 2111.52 ± 1255.88 days. 22 (81.4%) patients showed almost one infection during follow-up and before the development of BOS. Impaired lung function was observed in BOS. At the time of diagnosis of BOS, 24 patients were on tacrolimus and 22 on azithromycin. Less than 50% of patients were on cyclosporin and/or mycophenolate therapy. Regarding BAL analysis performed at the time of serum sampling, an increase in neutrophil count (28.91 ± 32.13) with respect to normal reference values (< 1) was recorded. An elevated CD8 + T cell count (63.24 ± 12.8) and a lower than normal CD4/CD8 ratio (0.68 ± 1.2) were recorded.
Serum Concentrations of COL4A5
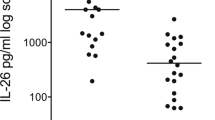
Serum concentrations of COL4A5 were higher in pre-BOS than in stable patients (40.5 ± 13.9 and 24.8 ± 11.4, respectively, p = 0.048), and lower in pre-BOS than BOS patients (40.5 ± 13.9 and 10.6 ± 3.34, respectively, p = 0.039) (Fig. 1a). Moreover, a direct correlation was found between the serum concentrations of COL4A5 pre-BOS and at the time of BOS diagnosis (r = 0.89 p = 0.001) (Fig. 1b).
Survival Analysis
ROC curve analysis of BOS and stable patients did not discriminate the two groups (p > 0.05); a cut-off value of 5.64 pg/ml was the best concentration in terms of specificity and sensitivity (57.14 and 55.56%, respectively). Comparing pre-BOS concentrations of COL4A5 and stable patients, the best cut-off point was 10.82 pg/ml with a specificity and sensitivity of 57.14 and 52.6%, respectively.
When our entire cohort was stratified on the basis of serum concentrations of COL4A5 (< 5.64 pg/ml and > 5.64 pg/ml), the former showed a higher probability of survival (p = 0.038) (Fig. 2a).
a Kaplan–Meier survival probabilities curves in LTX patients (41 patients) stratify based on COL4A5 concentrations (< 5.64 pg/ml). b Kaplan–Meier CLAD-free survival curves of patients with BOS stratify based on COL4A5 concentrations (< 5.64 pg/ml). c Kaplan–Meier CLAD-free survival curves of patients with BOS stratify based on COL4A5 concentrations before BOS concentrations (10.82 pg/ml)
No statistically significant difference in number of CLAD-free days emerged between the two groups (p = 0.548) (Fig. 2b).
When we stratified our BOS cohort on the basis of a pre-BOS COL4A5 cut-off value of 10.82 pg/ml (< 10.64 pg/ml and > 10.82 pg/ml), we observed that the former group had longer CLAD-free survival than the latter group (p = 0.046) (Fig. 2c).
Multinomial Logistic Regression
The logistic regression models were designed to explore the role of predictors that may affect serum concentrations of COL4A5. In the first two models, we performed binomial logistic regression and assigned our cohort scores of 0 and 1 on the basis of the two cut-offs (5.64 pg/ml and 10.82 pg/ml), using survival, type of transplant, acute rejection, infections, and therapies as intercepts. None of these variables influenced stratification of the groups (Suppl. Tables 1–2).
A third model was built using the two groups (Stable and BOS) as coefficients. In this case, serum COL4A5 (z score: 2.27, p = 0.02), COL4A5 pre-BOS (z score: − 2.17, p = 0.03), and overall survival (z score: 1.85, p = 0.06) influenced the development of BOS (Table 2). This means that BOS development impacts on COL4A5 levels both on COL4A5 at the moments of diagnosis and 3–6 months before clinical diagnosis.
Correlation Analysis
Figure 3a–c reports the correlation between functional parameters (FEV1, FVC, and DLCO expressed as percentages) and COL4a5 concentration at the moment of BOS diagnosis. Moreover patients were stratified for survival data (as live and dead).
a Spearman correlation analysis of FVC and COL4A5 concentrations at BOS diagnosis in patients stratifies as live and dead. b Spearman correlation analysis of Fev1 and COL4A5 concentrations at BOS diagnosis in patients stratifies as live and dead. c Spearman correlation analysis of FVC and COL4A5 concentrations pre-BOS diagnosis in patients stratifies as live and dead. d Δ correlation analysis between pulmonary functions and COL4A5 levels. e, f Spearman correlation analysis of FEV1 (e) and FVC (f) and COL4A5 concentrations at BOS diagnosis in patients stratifies as COL4A5 < 5.64 and COL4A5 > 5.64 pg/ml
This stratification showed an inverse correlation between serum concentration of COL4A5, predicted FVC (Fig. 3a) (r = − 0.56 p = 0.03), and FEV1 (Fig. 3b) (r = − 0.42 p = 0.04) at the time of diagnosis of BOS and reached particular statistical significance for patients who died.
When serum concentrations of COA4A5 pre-BOS were analysed, only FVC proved to be statistically significant for patients of the BOS group who died (r = − 0.52; p = 0.04) (Fig. 3c).
Differences (Δ) between serum concentrations of COL4A5 before and at diagnosis of BOS were calculated. ΔCOL4A5 showed a significant correlation with ΔFEV1 (r = 0.61 p = 0.005) and ΔFVC (r = 0.75 p = 0.003) but not with DLCO (Fig. 3d).
After the stratification of patients into two groups (group 0 with COL4A5BOS < 5.64 pg/ml and group 1 with [COL4A5]BOS > 5.64 pg/ml), an inverse correlation with FEV1 (r = − 0.65 p = 0.001) (Fig. 3e) and FVC (r = − 0.31 p = 0.048) (Fig. 3f) only emerged in the case of group 1.
Discussion
Bronchiolitis obliterans syndrome is a common complication of lung transplant and is a negative event for survival. Criteria for the diagnosis of BOS are defined by ISHLT guidelines [23] and include clinical and functional parameters such as FEV1, FVC, and DLco. Although the histological features of BOS, especially in the later stages of the disease, have been abundantly described, early diagnosis of BOS is still a challenge. For this reason, many studies have focused on biomarkers that could help clinicians predict patients at higher risk of developing BOS. Surveillance bronchoscopy, including bronchoalveolar lavage and transbronchial biopsy, is part of routine follow-up and involves invasive procedures often inadvisable in patients in poor condition. This is why we chose serum as a safe, readily obtained, and cost-effective biological matrix.
We investigated the role of serum concentrations of COL4A5 as a potential marker of BOS. Our results showed that already 3–6 months before diagnosis of BOS, peripheral concentrations of COL4A5 were higher in patients who developed BOS than in stable patients.
Serum concentrations of COL4A5 can also be considered a good prognostic marker due to their association with survival, being lower in survivors than in patients who died. In the deceased group, a correlation was demonstrated between the marker and impaired lung function. Interestingly, this protein is not influenced by comorbidities, such as acute rejection or infections, or by therapies for BOS.
From a biological point of view, the main biological triggers of BOS remain unclear. Self-perpetuating activation of fibroblasts and excessive release of collagen leading to peribronchiolar fibrosis after a decline in lung function and airway obstruction are reported to be associated with onset of BOS [25]. This led to research into several markers related to the extracellular matrix (ECM), such as matrix metalloproteinase-9 (MMP-9), and to tissue remodelling, such as VEGF and VEGF receptor 2 (VEGFR2) [26,27,28].
In particular, in COPD patients, ECM turnover may damage lung architecture, impairing lung function [16]. Matrix metalloproteinase breaks down different fragments of collagen. While patients chronic rejection showed small-airway inflammation known as “obliterative bronchiolitis” that leads to epithelial damage, airway fibrosis and remodelling of the ECM affect the lung interstitial matrix and basement membrane [29]. Some studies showed that besides their role in tissue remodelling, serum concentrations of different MMPs (especially MMP-9) were correlated with BAL neutrophilia in patients with BOS [30, 31]. Interestingly, our cohort of patients also showed BAL neutrophilia.
It has also been demonstrated that FEV1 percentages are inversely correlated with collagen concentration on the surface of the epithelial basement membrane, suggesting that increased bronchial deposition of collagen contributes to deteriorating lung function and airway remodelling [16]. In line with this finding, our data showed a correlation between serum concentrations of COL4A5 and FEV1 at the time of diagnosis of BOS.
The limits of our study include its monocentric and retrospective nature. However, it is the first study to investigate serum concentrations of COL4A5 in BOS patients at and before clinical diagnosis of BOS, and to propose COL4A5 as a potential predictive biomarker of BOS development and as a marker of negative prognosis in lung transplant patients.
References
Yeung JC, Keshavjee S (2014) Overview of clinical lung transplantation. Cold Spring Harb Perspect Med 4:a015628. https://doi.org/10.1101/cshperspect.a015628
Sato M (2013) Chronic lung allograft dysfunction after lung transplantation: the moving target. Gen Thorac Cardiovasc Surg 61:67–78. https://doi.org/10.1007/s11748-012-0167-3
Verleden GM, Raghu G, Meyer KC, Glanville AR, Corris P (2014) A new classification system for chronic lung allograft dysfunction. J Heart Lung Transplant 33:127–133. https://doi.org/10.1016/j.healun.2013.10.022
Verleden GM, Vos R, Verleden SE, De Wever W, De Vleeschauwer SI, Willems-Widyastuti A, Scheers H, Dupont LJ, Van Raemdonck DE, Vanaudenaerde BM (2011) Survival determinants in lung transplant patients with chronic allograft dysfunction. Transplantation 92:703–708. https://doi.org/10.1097/TP.0b013e31822bf790
Verleden SE, Von der Thüsen J, Roux A, Brouwers ES, Braubach P, Kuehnel M, Laenger F, Jonigk D (2020) When tissue is the issue: a histological review of chronic lung allograft dysfunction. Am J Transplant 20:2644–2651. https://doi.org/10.1111/ajt.15864
Weigt SS, DerHovanessian A, Wallace WD, Lynch JP, Belperio JA (2013) Bronchiolitis obliterans syndrome: the Achilles’ heel of lung transplantation. Semin Respir Crit Care Med 34:336–351. https://doi.org/10.1055/s-0033-1348467
Stewart S, Fishbein MC, Snell GI, Berry GJ, Boehler A, Burke MM, Glanville A, Gould FK, Magro C, Marboe CC et al (2007) Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant 26:1229–1242. https://doi.org/10.1016/j.healun.2007.10.017
King TE (1989) Bronchiolitis obliterans. Lung 167:69–93. https://doi.org/10.1007/BF02714935
Bergantini L, d’Alessandro M, Otranto A, Cavallaro D, Gangi S, Fossi A, Perillo F, Luzzi L, Zanfrini E, Paladini P et al (2022) Characterization of NKG2-A/-C, Kir and CD57 on NK cells stimulated with Pp65 and IE-1 antigens in patients awaiting lung transplant. Life (Basel) 12:1081. https://doi.org/10.3390/life12071081
Arjuna A, Olson MT, Walia R, Bremner RM, Smith MA, Mohanakumar T (2021) An update on current treatment strategies for managing bronchiolitis obliterans syndrome after lung transplantation. Expert Rev Respir Med 15:339–350. https://doi.org/10.1080/17476348.2021.1835475
Cavallaro D, Guerrieri M, Cattelan S, Fabbri G, Croce S, Armati M, Bennett D, Fossi A, Voltolini L, Luzzi L et al (2022) Markers of bronchiolitis obliterans syndrome after lung transplant: between old knowledge and future perspective. Biomedicines 10:3277. https://doi.org/10.3390/biomedicines10123277
Jaramillo A, Fernández FG, Kuo EY, Trulock EP, Patterson GA, Mohanakumar T (2005) Immune mechanisms in the pathogenesis of bronchiolitis obliterans syndrome after lung transplantation. Pediatr Transplant 9:84–93. https://doi.org/10.1111/j.1399-3046.2004.00270.x
Müller C, Rosmark O, Åhrman E, Brunnström H, Wassilew K, Nybom A, Michaliková B, Larsson H, Eriksson LT, Schultz HH et al (2021) Protein signatures of remodeled airways in transplanted lungs with bronchiolitis obliterans syndrome obtained using laser capture microdissection. Am J Pathol 191:1398–1411. https://doi.org/10.1016/j.ajpath.2021.05.014
Bergantini L, d’Alessandro M, Cavallaro D, Pordon E, Cassai L, Gangi S, Meloni F, Montagnani F, Paladini P, Refini RM et al (2012) Immune checkpoint analysis of T-cell responses to Pp65 and IE-1 antigens in end-stage lung diseases. Scand J Immunol. https://doi.org/10.1111/sji.13248
Gatseva A, Sin YY, Brezzo G, Van Agtmael T (2019) Basement membrane collagens and disease mechanisms. Essays Biochem 63:297–312. https://doi.org/10.1042/EBC20180071
Schumann DM, Leeming D, Papakonstantinou E, Blasi F, Kostikas K, Boersma W, Louis R, Milenkovic B, Aerts J, Sand JMB et al (2018) Collagen degradation and formation are elevated in exacerbated COPD compared with stable disease. Chest 154:798–807. https://doi.org/10.1016/j.chest.2018.06.028
Liesker JJW, Ten Hacken NH, Zeinstra-Smith M, Rutgers SR, Postma DS, Timens W (2009) Reticular basement membrane in asthma and COPD: similar thickness, yet different composition. Int J Chron Obstruct Pulmon Dis 4:127–135. https://doi.org/10.2147/copd.s4639
Menter DG, Dubois RN (2012) Prostaglandins in cancer cell adhesion, migration, and invasion. Int J Cell Biol 2012:723419. https://doi.org/10.1155/2012/723419
Santis, M.M.D.; Wagner, D.E. Collagen IV: A Critical New Starting Point for Engineering Upper Airways. European Respiratory Journal 2020, 55, doi:https://doi.org/10.1183/13993003.01130-2020.
Abreu-Velez AM, Howard MS (2012) Collagen IV in normal skin and in pathological processes. N Am J Med Sci 4:1–8. https://doi.org/10.4103/1947-2714.92892
Manninen A (2015) Epithelial polarity-generating and integrating signals from the ECM with integrins. Exp Cell Res 334:337–349. https://doi.org/10.1016/j.yexcr.2015.01.003
Mutgan AC, Jandl K, Kwapiszewska G (2029) Endothelial basement membrane components and their products, matrikines: active drivers of pulmonary hypertension? Cells 2020:9. https://doi.org/10.3390/cells9092029
Meyer KC, Raghu G, Verleden GM, Corris PA, Aurora P, Wilson KC, Brozek J, Glanville AR (2014) the ISHLT/ATS/ERS BOS Task Force Committee An International ISHLT/ATS/ERS clinical practice guideline: diagnosis and management of bronchiolitis obliterans syndrome. Eur Respir J 44:1479–1503. https://doi.org/10.1183/09031936.00107514
Stanojevic S, Kaminsky DA, Miller MR, Thompson B, Aliverti A, Barjaktarevic I, Cooper BG, Culver B, Derom E, Hall GL et al (2022) ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J 60:2101499. https://doi.org/10.1183/13993003.01499-2021
Colom AJ, Teper AM (2019) Post-infectious bronchiolitis obliterans. Pediatr Pulmonol 54:212–219. https://doi.org/10.1002/ppul.24221
Pain M, Royer P-J, Loy J, Girardeau A, Tissot A, Lacoste P, Roux A, Reynaud-Gaubert M, Kessler R, Mussot S et al (2017) T cells promote bronchial epithelial cell secretion of matrix metalloproteinase-9 via a C-C chemokine receptor type 2 pathway: implications for chronic lung allograft dysfunction. Am J Transplant 17:1502–1514. https://doi.org/10.1111/ajt.14166
Kennedy VE, Todd JL, Palmer SM (2013) Bronchoalveolar lavage as a tool to predict, diagnose and understand bronchiolitis obliterans syndrome. Am J Transplant 13:552–561. https://doi.org/10.1111/ajt.12091
Khatri P, Roedder S, Kimura N, De Vusser K, Morgan AA, Gong Y, Fischbein MP, Robbins RC, Naesens M, Butte AJ et al (2013) A common rejection module (CRM) for acute rejection across multiple organs identifies novel therapeutics for organ transplantation. J Exp Med 210:2205–2221. https://doi.org/10.1084/jem.20122709
Bos S, Milross L, Filby AJ, Vos R, Fisher AJ (2022) Immune processes in the pathogenesis of chronic lung allograft dysfunction: identifying the missing pieces of the puzzle. Eur Respir Rev 31:220060. https://doi.org/10.1183/16000617.0060-2022
Heijink IH, Rozeveld D, van der Heide S, van der Bij W, Bischoff R, van Oosterhout AJ, van der Toorn M (2015) Metalloproteinase profiling in lung transplant recipients with good outcome and bronchiolitis obliterans syndrome. Transplantation 99:1946–1952. https://doi.org/10.1097/TP.0000000000000602
Banerjee B, Ling K-M, Sutanto EN, Musk M, Yerkovich ST, Hopkins PMA, Stick SM, Kicic A, Chambers DC (2011) The airway epithelium is a direct source of matrix degrading enzymes in bronchiolitis obliterans syndrome. J Heart Lung Transplant 30:1175–1185. https://doi.org/10.1016/j.healun.2011.06.007
Acknowledgements
The Tuscany Transplant Group includes Luca Voltolini,2 Federico Franchi,3 Sabino Scolletta,3 Luca Luzzi,4 and Adriano Peris5.
Funding
Open access funding provided by Università degli Studi di Siena within the CRUI-CARE Agreement. No funding sponsors to declare.
Author information
Authors and Affiliations
Consortia
Contributions
AM and CS collected clinical data and wrote the paper; GM, MM, PB, and GM, collection of data, methodology, and supervision; d’AM, GS, perform experiment and statistical analysis, CP PF BD, and FA, cohort selection, supervision, interpretation of results, BE wrote and correction of paper, BL supervision, statistical analysis, and wrote and editing the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The present study was performed at Siena University.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Armati, M., Cattelan, S., Guerrieri, M. et al. Collagen Type IV Alpha 5 Chain in Bronchiolitis Obliterans Syndrome After Lung Transplant: The First Evidence. Lung 201, 363–369 (2023). https://doi.org/10.1007/s00408-023-00632-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-023-00632-8