Abstract
Background
There is very little evidence of the utility of the exhaled fraction of NO (FeNO) for the diagnosis of interstitial lung disease and nearly all of it is related with connective tissue disease. Some authors have suggested that in patients with hypersensitivity pneumonitis (HP), evolution to pulmonary fibrosis may be mediated by a Th2 mechanism, which could redound in a potential utility of FeNO. The aim of this study was to investigate the values of FeNO before and after antigenic exposure with the specific inhalation challenge (SIC) and to analyze its potential utility for the diagnosis of HP.
Methods
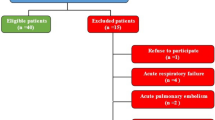
It was a prospective, cross-sectional study of all patients older than 18 years referred to our center for suspected chronic HP between May 2012 and May 2014 and who underwent a SIC. FeNO was collected before and after SIC.
Results
The study sample comprised 25 patients. Eleven were diagnosed with chronic HP; six had been exposed to avian proteins and five to fungal agents. Of these 11 patients, seven had positive SICs. In the 14 patients with diagnoses other than HP, all the SICs were negative. No significant differences in baseline characteristics were observed according to HP diagnosis, except in the BAL lymphocyte count. No differences were found after the test in patients diagnosed with HP; nor were there differences in baseline FeNO in patients diagnosed with HP and those who received alternative diagnoses.
Conclusions
The results suggest that FeNO measurement is not useful for the diagnosis of chronic HP.


Similar content being viewed by others
References
Costabel U, Bonella F, Guzman J (2012) Chronic hypersensitivity pneumonitis. Clin Chest Med 33(1):151–163
Schuyler M, Cormier Y (1997) The diagnosis of hypersensitivity pneumonitis. Chest 111(3):534–536
Bjermer L, Alving K, Diamant Z et al (2014) Current evidence and future research needs for FeNO measurement in respiratory diseases. Respir Med 108(6):830–841
Villar A, Muñoz X, Sanchez-Vidaurre S et al (2014) Bronchial inflammation in hypersensitivity pneumonitis after antigen-specific inhalation challenge. Respirology 19(6):891–899
Chow S, Thomas PS, Malouf M et al (2012) Exhaled breath condensate (EBC) biomarkers in pulmonary fibrosis. J Breath Res 6(1):016004
Ojanguren I, Cruz MJ, Villar A et al (2015) Changes in pH exhaled breath condensate after specific inhalation bronchial challenge test in patients with hypersensitivity pneumonitis: a prospective study. BMC Pulm Med 15:109
Barnes PJ, Dweik RA, Gelb AF et al (2010) Exhaled nitric oxide in pulmonary diseases: a comprehensive review. Chest 138(3):682–692
Kharitonov SA, Gonio F, Kelly C et al (2003) Reproducibility of exhaled nitric oxide measurements in healthy and asthmatic adults and children. Eur Respir J 21(3):433–438
Bazeghi N, Gerds TA, Budtz-Jørgensen E et al (2011) Exhaled nitric oxide measure using multiple flows in clinically relevant subgroups of COPD. Respir Med 105(9):1338–1344
Saito J, Gibeon D, Macedo P et al (2014) Domiciliary diurnal variation of exhaled nitric oxide fraction for asthma control. Eur Respir J 43(2):474–484
Kersul AL, Iglesias A, Ríos Á et al (2011) Molecular mechanisms of inflammation during exacerbations of chronic obstructive pulmonary disease. Arch Bronconeumol 47(4):176–183
Suresh V, Mih JD, George SC (2007) Measurement of IL-13-induced iNOS-derived gas phase nitric oxide in human bronchial epithelial cells. Am J Respir Cell Mol Biol 37(1):97–104
Wenzel S, Wilbraham D, Fuller R et al (2007) Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: results of two phase 2a studies. Lancet 370(9596):1422–1431
Ricciardolo FL, Sterk PJ, Gaston B et al (2004) Nitric oxide in health and disease of the respiratory system. Physiol Rev 84(3):731–765
Barrera L, Mendoza F, Zuñiga J et al (2008) Functional diversity of T-cell subpopulations in subacute and chronic hypersensitivity pneumonitis. Am J Respir Crit Care Med 177(1):44–55
Guilleminault L, Saint-Hilaire A, Favelle O et al (2013) Can exhaled nitric oxide differentiate causes of pulmonary fibrosis? Respir Med 107(11):1789–1796
Morell F (2013) Idiopathic pulmonary fibrosis: importance of accurate diagnosis and treatment. Arch Bronconeumol 49(8):319–320
Miller MR, Crapo R, Hankinson J et al (2005) ATS/ERS Task Force. General considerations for lung function testing. Eur Respir J 26(1):153–161
García-Río F, Calle M, Burgos F et al (2013) Spanish Society of Pulmonology and Thoracic Surgery (SEPAR). Spirometry. Spanish Society of Pulmonology and Thoracic Surgery (SEPAR). Arch Bronconeumol 49(9):388–401
Morell F, Roger A, Cruz MJ et al (2003) Suberosis: clinical study and new etiologic agents in a series of eight patients. Chest 124(3):1145–1152
Munoz X, Morell F, Cruz MJ (2013) The use of specific inhalation challenge in hypersensitivity pneumonitis. Curr Opin Allergy Clin Immunol 13(2):151–158
Morell F, Roger A, Reyes L et al (2008) Bird fancier’s lung: a series of 86 patients. Medicine (Baltimore). 87(2):110–130
Muñoz X, Sánchez-Ortiz M, Torres F et al (2014) Diagnostic yield of specific inhalation challenge in hypersensitivity pneumonitis. Eur Respir J 44(6):1658–1665
American Thoracic Society; European Respiratory Society (2005) ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med 171:912–930
Costabel U (1988) The alveolitis of hypersensitivity pneumonitis. Eur Respir J 1(1):5–9
Schuyler M, Gott K, Edwards B (1999) Th1 cells that adoptively transfer experimental hypersensitivity pneumonitis are activated memory cells. Lung 177(6):377–389
Yamasaki H, Ando M, Brazer W et al (1999) Polarized type 1 cytokine profile in bronchoalveolar lavage T cells of patients with hypersensitivity pneumonitis. J Immunol. 163(6):3516–3523
Gudmundsson G, Monick MM, Hunninghake GW (1998) IL-12 modulates expression of hypersensitivity pneumonitis. J Immunol 161(2):991–999
Mroz RM, Korniluk M, Stasiak-Barmuta A et al (2008) Upregulation of Th1 cytokine profile in bronchoalveolar lavage fluid of patients with hypersensitivity pneumonitis. J Physiol Pharmacol 59(Suppl 6):499–505
Ye Q, Nakamura S, Sarria R et al (2009) Interleukin 12, interleukin 18, and tumor necrosis factor alpha release by alveolar macrophages: acute and chronic hypersensitivity pneumonitis. Ann Allergy Asthma Immunol 102(2):149–154
Selman M, Pardo A, Barrera L et al (2006) Gene expression profiles distinguish idiopathic pulmonary fibrosis from hypersensitivity pneumonitis. Am J Respir Crit Care Med 173(2):188–198
Joshi AD, Fong DJ, Oak SR et al (2009) Interleukin-17-mediated immunopathogenesis in experimental hypersensitivity pneumonitis. Am J Respir Crit Care Med 179(8):705–716
Simonian PL, Roark CL, Wehrmann F et al (2009) Th17-polarized immune response in a murine model of hypersensitivity pneumonitis and lung fibrosis. J Immunol 182(1):657–665
Mitaka K, Miyazaki Y, Yasui M et al (2011) Th2-biased immune responses are important in a murine model of chronic hypersensitivity pneumonitis. Int Arch Allergy Immunol 154(3):264–274
Taylor DR, Mandhane P, Greene JM et al (2007) Factors affecting exhaled nitric oxide measurements: the effect of sex. Respir Res 8:82
McSharry CP, McKay IC, Chaudhuri R et al (2005) Short and long-term effects of cigarette smoking independently influence exhaled nitric oxide concentration in asthma. J Allergy Clin Immunol 116(1):88–93
Alving K, Minovschi A (2010) Basic aspects of exhaled nitric oxide. Eur Respir Monogr 49:1–31
Hanak V, Golbin JM, Ryu JH (2007) Causes and presenting features in 85 consecutive patients with hypersensitivity pneumonitis. Mayo Clin Proc 82(7):812–816
Xaubet A, Ancochea J, Morell F et al (2004) Spanish Group on Interstitial Lung Diseases, SEPAR. Report on the incidence of interstitial lung diseases in Spain. Sarcoidosis Vasc Diffuse Lung Dis 21(1):64–70
Acknowledgments
This study was supported by FIS PI13/01076 (Instituto de Salud Carlos III) and Sociedad Española de Patología Respiratoria (SEPAR, Spanish Society of Respiratory Disease). MJC is a researcher supported by the Miguel Servet programme from Instituto de Salud Carlos III (CP12/03101). The funders had no role in the study design, data collection or analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors have no competing interests to declare.
Rights and permissions
About this article
Cite this article
Ojanguren, I., Cruz, M.J., Villar, A. et al. Utility of Exhaled Nitric Oxide Fraction for the Diagnosis of Hypersensitivity Pneumonitis. Lung 194, 75–80 (2016). https://doi.org/10.1007/s00408-015-9824-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-015-9824-5