Abstract
Adult attention-deficit/hyperactivity disorder (aADHD) represents a heterogeneous entity incorporating different subgroups in terms of symptomatology, course, and neurocognition. Although neurocognitive dysfunction is generally associated with aADHD, its severity, association with self-reported symptoms, and differences between subtypes remain unclear. We investigated 61 outpatients (65.6% male, mean age 31.5 ± 9.5) diagnosed using DSM-5 criteria together with age-, sex-, and education-matched healthy controls (HC) (n = 58, 63.8% male, mean age 32.3 ± 9.6). Neurocognitive alterations were assessed using the Cambridge Neuropsychological Test Automated Battery (CANTAB) and compared between groups using the generalized linear model (GLM) method. Multivariate effects were tested by principal component analysis combined with multivariate pattern analysis. Self-reported symptom severity was tested for correlations with neurocognitive performance. GLM analyses revealed nominally significant differences between the aADHD and HC groups in several domains, however, only the Rapid Visual Information Processing measures survived correction, indicating impaired sustained attention and response inhibition in the aADHD group. Comparison of the predominantly inattentive and the hyperactive-impulsive/combined subtypes yielded nominally significant differences with higher levels of dysfunction in the inattentive group. In the stepwise discriminant analysis aADHD and HC groups were best separated with 2 factors representing sustained attention and reaction time. We found only weak correlations between symptom severity and CANTAB factors. aADHD patients are neuropsychologically heterogeneous and subtypes show different neurocognitive profiles. Differences between the aADHD and HC groups were driven primarily by the inattentive subtype. Sustained attention and its factor derivative showed the most significant alterations in aADHD patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Attention deficit-hyperactivity disorder (ADHD) is a childhood-onset neurodevelopmental disorder. It is characterized by the core symptoms of inattention, impulsivity and motor hyperactivity [1], which can lead to decreased educational performance and social difficulties in children, and by persistence of symptoms, somatic and psychiatric comorbidity in adults [2,3,4]. The etiology of ADHD, although not fully understood, is considered multifactorial; common and rare genetic variants together with environmental effects are associated with increased disease risk, worse symptom profiles and severity in later life [5]. ADHD persists into adulthood in 35–50% of cases [6, 7], resulting in an adulthood prevalence around 2.5% worldwide [8, 9]. Adult ADHD (aADHD) is characterized by transitioned symptom presentation and functional consequences, including decreased higher education and work outcomes, interpersonal and family dysfunction. Moreover, in 30% and 18% of cases substance misuse [10] and criminal involvement [11] are also reported, respectively. Therefore, ADHD represents a major public health issue that affects a considerable patient population and their environments, both in children and adults. As a consequence, it needs to be targeted with complex clinical and psychosocial modalities and considered in the lifespan perspective [12,13,14]. Importantly, symptom severity, course and prognosis can be improved by efficient pharmacological and non-pharmacological interventions, given that the disorder is identified, diagnosed, and treated in proper time [15].
Neurocognitive impairment has been consistently described in aADHD in the domains of attention and sustained attention, reaction time variability, motor inhibition, and different subdomains of executive functioning, including cognitive flexibility, working memory, and delay discounting. However, cognitive heterogeneity has also been demonstrated by a systematic analysis, identifying a subgroup of aADHD patients without any significant, easily measurable cognitive dysfunction [16]. Alterations in response inhibition as the hypothesized main driver of deficiencies in ADHD [17], have been only supported partially by empirical data. This led to the refinement of the unifying neuropsychological models of ADHD, e.g., Sonuga-Barke described the dual-pathway model [18], where executive dysfunction and alterations of the reward circuit, i.e., delay aversion, together give rise to the complex symptomatology of ADHD. Indeed, executive dysfunction seems to be an overlapping phenotype in all age groups and phenotypic subgroups of ADHD, while delay aversion is a potential driver of impulsive behavior [19]. Another potential source of heterogeneity in adults compared to children, is more developed and individually varying compensation strategies. The clinical importance of neurocognitive impairment is underscored by longitudinal results, showing that persistence into and decreased functionality in adulthood is predicted by the severity of neuropsychological alterations [20, 21]. Recently, Onandia-Hinchado et al. reviewed all available studies investigating cognitive impairments in aADHD [22], showing the involvement of both attentional and multiple executive domains.
Decision making as a proxy for risk taking and impulsivity has also been investigated in aADHD, with a special emphasis on the association of delay discounting alterations and impulsivity as core symptoms. Impaired delay discounting has been proposed as an important feature of aADHD, but the results remain conflicting. Pollak et al. [23] demonstrated similar levels of risk taking with the Cambridge Gambling Test, indicative of equal sensitivity to risk in the aADHD and healthy control groups. In a recent review, the same group [24] showed that not risk taking per se, rather decision-making strategies are affected in aADHD, leading to real-life risk taking behaviors.
The Cambridge Neuropsychological Test Automated Battery (CANTAB) is used extensively for the assessment of neurocognitive performance in several mental disorders [25, 26]. The applied subtests of the CANTAB software are independent of culture and measure distinct neurocognitive functions including psychomotor speed, sustained attention, visual memory, executive functions, working memory and planning, semantic/verbal memory, decision making, response control, and social cognition. Application of the CANTAB software in aADHD demonstrated similar degree of heterogeneity of neurocognition within patients as previous studies. In a group of 474 DSM-IV diagnosed aADHD patients and 163 healthy controls Fried et al. [27] demonstrated that despite the differences of 7 CANTAB subtests in the fields of working memory and executive functioning, these results failed to discriminate effectively between patients and healthy controls. However, the CANTAB results were helpful in identifying the patient subgroup with executive functioning disorder, a finding with potential implications for the clinical management of aADHD patients. Therefore, we can conclude that the CANTAB software is not suitable for diagnosing aADHD, primarily because the diagnostic criteria are based on behavioral symptoms, and do not fully encompass cognitive alterations. However, the CANTAB software, due to the growing level of complexity in certain subtests, which necessitate adaptability and executive functions in subjects, can delineate subgroups within aADHD characterized by more profound cognitive dysfunction. This unique feature warrants interest for continued studies of aADHD with the same approach.
It remains unclear to what extent different cognitive domains exert independent effects, i.e., are the different alterations specific and non-overlapping in their consequences on outcomes and symptom severity. Interestingly, inconsistencies that have been shown in the above studies could also be explained by differential neurocognitive alterations in the subtypes of aADHD. The distinction between the predominantly inattentive (aADHD-I), predominantly hyperactive-impulsive (aADHD-HI), and combined presentation subtypes (aADHD-C) of aADHD, as defined by DSM-5 [1] is made according to symptom severity of these domains. In children and youth, Nikolas and Nigg [28] found that the neuropsychological performance of the ADHD-C group was worse than the ADHD-I group. However, earlier in a meta-analysis of 83 studies of childhood ADHD no differences were revealed in executive functions between the combined and inattentive subtypes [29]. In adults, Dobson-Patterson et al. [30] found that the aADHD-I group showed a clear separation in a multivariate comparison of tests assessing attention, memory and executive function, while the aADHD-C group did not separate from the healthy control group using the same approach. In contrast, Phillips et al. demonstrated worse performance in the domains of visual and verbal memory in aADHD-C individuals [3, 4, 31]. These findings are suggestive of the hypothesis that in aADHD inattentive patients are affected more severely, but only in certain domains of neurocognitive impairment. A complementary approach to group comparisons is to test for associations between neurocognitive alterations and clinical variables, such as self-reported symptom severity or comorbidities. In this regard there is a scarcity of data in the literature, however, the existing results have shown that subjective and objective measures of cognitive impairments do not show good correlation [32].
This study had three aims: First we sought to describe neurocognitive alterations and decision making in aADHD and its subtypes, compared to healthy controls, in a sample of Hungarian patients. Second, using a factor-analytic approach as a data-reduction technique we compared aADHD subtypes in terms of neurocognitive alterations after controlling for overlapping effects and using a stepwise multivariate pattern analysis. Therefore, non-specific, overlapping effects between cognitive tests were also considered. Third, we analyzed the correlations of self-reported symptom severity with neurocognition. The rationale behind this complex approach is to decrease the phenotypic heterogeneity characteristic of aADHD, by investigating differences in disease subgroups and associations with subjective symptom severity as a continuous variable. Thus, a better understanding of the associations between neurocognitive dysfunction and aADHD can be reached.
Methods
Sample recruitment and characteristics
Sixty-one aADHD patients (40 male, 21 female) and fifty-eight healthy control individuals (37 male, 21 female) matched for sex, age, and educational level were included in the study (Table 1). All examinations were carried out between January 2016 and June 2017. aADHD patients were recruited at the adult ADHD outpatient clinic of the Department of Psychiatry and Psychotherapy, Semmelweis University, Budapest, Hungary. The diagnosis of aADHD was originally established according to DSM-IV-R diagnostic criteria, later all data were reanalyzed using the DSM-5 as well, demonstrating that all patients fulfill the criteria of mid-severe aADHD. The diagnosis and aADHD subtypes were established by two experienced psychiatrists based on DSM-5 criteria using a detailed clinical interview and patient history. Screening for psychiatric comorbidities was carried out using the Hungarian versions [33, 34] of the MINI PLUS 5.0 [35, 36] and SCID-II [37] interviews. Exclusion criteria in the patient group were IQ under 70, a comorbid diagnosis of neurocognitive disorders, psychotic disorders, and severe neurological conditions. 59 of the ADHD patients were medicated, with 58 receiving methylphenidate; and 1 patient receiving bupropion. Patients taking stimulant treatment were off medication at least 24 h before testing.
Healthy controls with negative psychiatric history were recruited from staff members, students and their acquaintances. They underwent a screening procedure and were excluded in case of positive neurological, psychiatric, or substance use disorder history, or a T-score above 70 on the Symptom Checklist 90-R (SCL-90-R) [38, 39]. Exclusion criteria in both groups were a positive history of severe head trauma and visual or movement impairment that could influence results during touch screen use. The study complied with the ethical standards of the Declaration of Helsinki, and received approval from the Hungarian Health Sciences Council Ethical Committee (IF-11390-8/2015 and IF-621-2/2017, modified by nrs. 25329-5/2018/EÜIG and 926-5/2018/EÜIG, respectively). All participants gave written informed consent.
Examination procedure, clinical and neuropsychological variables
After written informed consent, participants in both groups were asked to complete two self-report questionnaires: the Conner’s Adult ADHD Rating Scale (CAARS) [40] and the Symptom Check List (SCL-90-R) [38, 39]. The Hungarian version of the CAARS 66 item self-report questionnaire was used to assess symptom severity [40,41,42,43]. The CAARS measures the severity of self-reported symptoms on seven subscales (CAARS-A: Inattention/Memory Problems; CAARS-B: Hyperactivity/Restlessness; CAARS-C: Impulsivity/Emotional Lability; CAARS-D: Problems with Self-Concept; CAARS-E: DSM-IV Inattentive Symptoms; CAARS-F: DSM-IV Hyperactive-Impulsive Symptoms, CAARS-G: DSM-IV Total ADHD Symptoms; CAARS-H: ADHD Index). All items represent a 4-point scale (values between 0 and 3). The Hungarian version of the SCL-90-R [38, 39, 44, 45] was used to assess general psychopathology. Healthy controls were excluded if they tested above the T-score of 70 in the Derogatis criteria. IQ scores were assessed only in patients using the WAIS-R [46].
Cambridge Neuropsychological Test Automated Battery
All subjects underwent neurocognitive assessment using the Cambridge Neuropsychological Test Automated Battery (CANTAB EclipseTM 5.0, Cambridge Cognition, Cambridge, United Kingdom). CANTAB was originally developed to assess neurocognitive performance mainly of patients suffering from neurocognitive impairment [25, 26, 47]. Recently, it has been used to study several psychiatric disorders, and has been validated on several patient groups, resulting in good validity and reliability data. As of now, CANTAB has been used and proved to be a useful tool to assess cognitive functions in diverse neurological and psychiatric disorders, such as dementia, schizophrenia, and depression. The tests of CANTAB are independent of culture and measure several neurocognitive functions and domains including visual memory, executive function, attention, semantic/verbal memory, decision making and social cognition. The Hungarian version was first described by Bartók et al. in 2001 [48]. The first two tests (Motor screening, MOT, and Big-Little Circle, BLC) were used as screening tests to assess the subjects’ mental and physical suitability for the examination procedure. After these, the following tests were administered: Reaction Time (RTI), Intra-Extradimensional Shifting (IED), Rapid Visual Information Processing (RVP), Stockings of Cambridge (SOC), Spatial Working Memory (SWM), Spatial Span (SSP), Paired Associates Learning (PAL), and Cambridge Gambling Test (CGT) (Table 2). These tests were selected on the basis of previous results showing their sensitivity in ADHD, and the availability of the tests in the Hungarian version of CANTAB.
Statistical procedures
Statistical analyses were performed using SPSS version 22.0 (SPSS Inc., Chicago, Illinois, USA) [49] and SAS 9.4. (SAS Institute, Cary NC) [50]. Comparison of continuous and categorical demographic variables was carried out by the independent samples t-test and Chi-square test, respectively. The comparison of CANTAB variables between two (aADHD and HC) or three (aADHD subtypes and HC) groups was performed in two steps: first, raw data were used, and generalized linear model (GLM) analyses were performed, with gender, age and education level considered as confounders. The FDR method was applied to correct for multiple comparisons. To compare effect sizes of cognitive performance measures between the aADHD and HC groups, Cohen-d values were calculated, which were used to rank output variables.
Since neurocognitive variables measured with CANTAB are not independent from each other, we combined a factor-analytic approach including multiple CANTAB variables for each task and multivariate pattern analysis, with the aim of data-reduction. This multivariate approach complements the above described univariate comparisons. Factor analysis with varimax rotation was used on all variables except for BLC, MOT and SSP, factors with an Eigenvalue above 1 were retained [51]. CANTAB variables with an absolute factor loading over 0.7 were identified, and were assigned to one of the factors based on the highest factor loading. Variables assigned to the same factor were collapsed together to a canonical variable. Next, we conducted multi-group confirmatory factor analysis (MGCFA) to assess whether the structure of latent cognitive traits was similar in patients and controls [52,53,54].
These canonical variables served as input to stepwise discriminant models to find the most significant factors that differentiate study groups. During this multivariate pattern-analysis the forward selection begins with no variables in the model. At each step, the model enters the variable that contributes most to the discriminatory power of the model as measured by Wilks’s lambda, the likelihood ratio criterion. When none of the unselected variables meet the entry criterion, the forward selection process stops. Finally, to explore possible associations between neurocognitive variables and subjective symptom severity, Pearson’s correlations were calculated between canonical factors and CAARS subscores in the aADHD sample.
Results
Clinical and demographic data
Basic demographic and clinical characteristics of the aADHD and HC groups are demonstrated in Table 1. The groups were comparable in sex, age and educational level. Assessment of comorbidities revealed that more than half of the ADHD patients had at least one other psychiatric diagnosis, most of them suffering from major depressive disorder and anxiety disorders. The aADHD group had higher severity of general psychopathology in all variables, as measured by the SCL-90-R scale, and as expected, patients displayed higher severity on all CAARS symptom dimensions, including inattention, hyperactivity, impulsivity, problems with self-concept, and on all specific, DSM-based symptom scales. Mean IQ score of the aADHD group was 125.8 (SD = 12.12).
Comparison of neurocognitive performance between the aADHD and HC groups
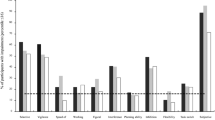
First, we made comparisons between the aADHD and the healthy control groups’ cognitive performance measured by the CANTAB battery (Table 3). These results showed decreased cognitive performance in the aADHD group impacting the following domains and task variables: sustained attention (RVP, RVP A′, Probability of hit, Mean latency, Total correct rejection, Total misses), spatial working memory (SWM, Strategy, Total errors, Between errors), working memory capacity (SSP, Span length, Total errors), visual memory (PAL, Mean trials to success), reaction time (RTI, Five-Choice reaction time, Five-choice accuracy score), and the latency measure of the cognitive flexibility/set-shifting task (IED, Total latency). The between-group differences in these variables were nominally significant, resulting in moderate effect sizes with Cohen-d values between 0.38 and 0.62 (Fig. 1), but only the sustained attention domain RVP-variables survived FDR-correction for multiple comparisons (p = 0.037). We found no differences between groups in other variables.
Cohen-d values of the most important CANTAB variables based on comparisons between the aADHD and HC groups in the order of absolute values RVP rapid visual information processing, IED intra/extradimensional shifting, SWM spatial working memory, SSP spatial span, RTI reaction time, PAL paired association learning, BLC big/little circle, CGT Cambridge gambling task
Comparison of neurocognitive performance between aADHD subtypes
Next, we performed comparison between the aADHD subgroups and healthy controls (HC) using GLM analyses. The subgrouping was based on the presence of DSM-5 hyperactive-impulsive threshold. Because of the small number of purely hyperactive-impulsive patients, we investigated two patient subgroups, the inattentive group (aADHD-I) and the mixed group (aADHD-MIX) consisting of aADHD-HI and aADHD-C patients. The between-subtype comparisons revealed differences reaching nominal significance in several cognitive domains and CANTAB test variables (Table 4). The affected domains were sustained attention (RVP A′, probability of hit, total correct rejections, total misses, mean latency), working memory (SWM, strategy, total errors, between errors, SSP, span length, mean time for first response), and cognitive flexibility (IED, total latency). Post-hoc analyses showed decreased cognitive performance in aADHD-I compared to HC in RVP, SWM, and CGT variables. Comparisons between aADHD-MIX and HC groups yielded nominally significant results in the IED, SWM, RVP, RTI and SSP tests. None of these between-subtype differences survived FDR correction for multiple comparisons.
Multivariate pattern analysis based on principal component and step-wise discriminant analysis
As a data-reduction technique, we used principal component analysis. Factor analysis of 46 variables yielded 12 factors with an eigenvalue higher than one. Thirty-one variables were assigned to one of the factors based on the absolute value of factor loadings above 0.7 (Table 5). The identified factors separated exclusively according to CANTAB tests, i.e., we found no factor loaded by different CANTAB subtests. Four of these factors were excluded from downstream analyses because they were only loaded by one variable. Multi-group confirmatory factor analysis performed as a quality-control step suggested 11 factors as optimal. The structure of three factors were questioned at this quality-control step. The IED1 factor was also loaded by “IED Completed stage trials and errors” in both the aADHD and control groups. The SWM2 factor was also loaded with “SOC Mean initial thinking time 5 moves” in the HC group, and the RTI1 “Mean simple movement time” was replaced with “SWM Within errors” variable in the aADHD group. Figure 2 summarizes results of the multivariate pattern-analysis procedures.
Summary of principal component and multivariate pattern analysis procedures. The CANTAB variables used in the multivariate pattern analysis are shown on the left. Colored lines represent factor absolute loadings above 0.7 (red indicates positive loads, while blue indicates negative loads) to the 12 factors presented in the middle column. Short descriptions of factors are presented under factor abbreviations. Finally, connections between the factors and the 3 separate step-wise discriminant analyses are highlighted on the right side. RVP rapid visual information processing, IED intra/extradimensional shifting, SWM spatial working memory, SSP spatial span, RTI reaction time, PAL paired association learning, BLC big/little circle, CGT Cambridge gambling task
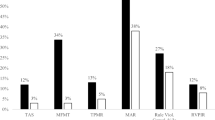
Comparison of the remaining 8 canonical variables demonstrated significant differences between aADHD and HC groups in the RVP1 and SWM1 factors (p < 0.02 after FDR correction), representing the domains of sustained visual attention and spatial working memory (Fig. 3 and Table 6). Canonical variables derived for each factor were used for performing stepwise discriminant analysis. First, healthy controls and the aADHD group were best separable with 2 factors, namely RVP1 and RTI1. Next, the discrimination between all the three groups was tested resulting in SWM1, RVP1 and RTI1 as discriminating factors. Finally, we repeated the analysis to identify factors that could differentiate between aADHD subtypes most efficiently. This analysis yielded 2 discriminating factors, RTI1 and IED1 (Table 7 and Supplementary Table 1).
Correlations between CANTAB-based canonical variables and self-reported symptom severity
Finally, correlation analyses were carried out between the self-reported symptom severity variables measured by CAARS, and the cognitive domains based on canonical variables. We found only scattered correlations demonstrating nominal statistical significance, with weak to moderate correlation coefficients. After correction for sign (necessary because of the inverse scoring in this test), the RVP1 factor showed correlations with the CAARS-C and CAARD-D subscales, the PAL factor was correlated with CAARS-A, while the RTI2 factor had the most positive correlations with different CAARS subscales. An overview of correlation coefficients is presented as a heatmap in Fig. 4.
Heatmap demonstrating correlations of self-reported symptom severity measured by CAARS and neurocognition factors in the whole sample. Hot colors show positive, while cold colors indicate negative correlation. Colors are scaled between − 1 and 1. Rows represent psychopathology measures with CAARS subscores and columns represent factor based canonical variables
Discussion
This study aimed to compare neuropsychological functioning between groups of adults living with ADHD and healthy controls, as well as between aADHD subtypes. Moreover, we sought to investigate the relationship between self-reported symptom severity and neurocognition in a thoroughly characterized clinical sample. To our knowledge, the current study is the first to use CANTAB to examine the neuropsychological characteristics of ADHD in a sample of Hungarian adults, a country representing Eastern-Central Europe, a region where aADHD is still underdiagnosed compared to Western European countries, due to the distinct developmental course of the mental health care system during past decades.
Comparison of adults diagnosed with ADHD to matched healthy controls yielded significant differences in certain CANTAB subtests indicative of decreased performance in attention and psychomotor speed (sustained visual attention, signal detectability, alertness, processing speed, shifting and flexibility of attention), executive functions (spatial working memory), and memory (short-term spatial memory, visual memory). In domains of higher executive functions (spatial planning and problem solving, decision making and risk-taking behavior) we could not observe differences between the two groups. It is important to note that after FDR-correction only the RVP subtest variables measuring sustained visual attention, alertness, and signal detectability remained significant.
It is conceivable that the significant differences at the RVP subtest reflect decreased sustained attention capacity, which aADHD patients are not able to improve by compensation and adaptation strategies, due to the unique characteristics of this test, such as time-pressure and duration. While compensation strategies, such as increased focusing may improve performance in other subtests, they have a very limited effect in the RVP subtest. In line with this interpretation both two- and three-group comparisons in our sample indicated increased mean latency and total miss values in the aADHD groups compared to the HC group. Indeed, sustained attention has been shown to be the most sensitive marker of vigilance dysfunction in different modalities, including neuropsychological [55, 56], functional brain imaging [57], and electrophysiology studies [58]. It has been suggested that deficits in sustained attention potentially reflect catecholamine (dopamine and norepinephrine) dysregulation [59, 60]. In the other tests (SWM, SSP, PAL, IED) only nominally significant differences were detectable, which did not survive FDR correction. These less pronounced differences can be explained by cognitive compensation strategies, which are typical for adults with ADHD, but less developed and observable in childhood ADHD [61]. Overall, in line with previous studies [27], our findings show that neuropsychological testing with the CANTAB software is helpful at identifying deficits in individual patients at specific cognitive domains like attention, executive function, memory, but cannot provide a clear diagnostic reference point and cutoff scores to properly separate patients and healthy controls. Based on our results, the RVP test is the most reliable for discriminating the two groups.
Neurocognitive heterogeneity has been shown not only across, but also within studies of aADHD [62], therefore it seems essential to investigate whether there are differences in cognitive performance between aADHD symptom-based subtypes. According to our results, the performance of the aADHD-I and aADHD-MIX groups are both significantly different when compared to healthy controls, but the implicated functions are divergent. Overall, adults with predominantly inattentive symptoms showed more deviations compared to matched controls than individuals with mixed symptoms, namely difficulties in spatial working memory (SWM, strategy, total and between errors) and visual memory (PAL, mean trials to success). In comparison, adults with mixed symptoms showed difficulties in short-term spatial memory, and both aADHD groups showed poorer performance in sustained attention and signal detectability variables (RVP). Mean latency was increased in both subtypes, however in aADHD-MIX patients this was paralleled with better accuracy (probability of hit) and lower number of total misses, despite the impulsive symptoms. The pattern of alterations can be interpreted as a partially successful compensation strategy of the aADHD-MIX individuals. These findings, although based on only nominally significant differences, support the hypothesis that individuals with aADHD-I can be affected more severely by neurocognitive deficits. In children, results remain diverse in this regard [28, 29].
It is an interesting question why cognitive performance is not affected as severely in the aADHD-MIX group where attention deficit is also present at the symptomatic level, and why results do not reflect the symptoms of impulsivity and hyperactivity. For example, response inhibition measured by probability of false alarm, commission errors in the RTI task, or alterations of decision making in the CGT tasks were unchanged, however RVP mean latency was affected in the aADHD-MIX group as well. We can hypothesize that motor impulsivity is better compensated in the combined subtype, and subjective attentional problems can be the results of secondary phenomena associated with increased compensation demand and impulsive traits.
Previous studies exploring the structure of CANTAB variables with a factor-analytic approach used only a limited number of CANTAB variables, investigating usually only one raw variable per task [63, 64]. Since multiple variables may characterize specific aspects of the subtest, we carried out a multivariate pattern analysis on multiple CANTAB variables. Multi-group confirmatory factor analysis showed preserved factor structure in both groups compared to pooled subjects. Using stepwise discriminant analysis differentiation between aADHD and controls was best among factors measuring sustained visual attention, signal detectability and arousal (RVP1) and psychomotor speed (RTI1), while separating three groups was best achieved with the inclusion of the spatial working memory (SWM1) factor to the above factors. The two aADHD subtypes were best separable with RTI1 and a factor measuring primarily set-shifting and flexibility of attention (IED1). The combination of RVP1 and RTI1 can be interpreted as sustained attention deficit and emergent compensatory mechanisms, moreover, the differences at IED1 between aADHD subtypes represent the deficits of cognitive flexibility necessary to dynamically adjust attention and behavior in various situations by monitoring and compensating performance [65, 66]. As expected, the multivariate comparisons applying data-reduction techniques yielded more significant results between groups, than head-to-head comparisons of raw neuropsychological variables. For example, both two- and three-group comparisons demonstrated differences both at the RVP1 and SWM1 factors, while in head-to-head comparisons only RVP variables survived correction for multiple comparisons.
Correlations between DSM-based symptom severity and performance on neuropsychological tests, as reflected by factors were generally low. Our findings showed that higher inattention and memory problems in the CAARS assessment scores were related to slower reaction time and difficulties in shifting, reflected by higher scores of the RTI2 factor. In addition, higher levels of impulsivity, emotional lability and problems with self-concept reported in CAARS scores were associated with worse sustained visual attention, signal detectability and arousal, reflected by lower scores of the RVP1 factor. No other significant correlations were found. The present results are in line with findings from previous studies [31], demonstrating low levels of correlation between self-reported complaints and objective neurocognitive performance. Adding to the observed heterogeneity, it has been shown recently that late-onset aADHD patients have a distinct neurocognitive profile with less impairment in alertness and executive functioning [67]. These findings support the hypothesis that cognitive profile can be more closely related to functional impairments than to symptoms per se [62]. The differences between the aADHD and HC groups in the subjective and neuropsychological tests as well, and the fact that they were loosely associated, suggests that both are necessary during the assessment of symptoms, these screening methods are not interchangeable. According to a recent study conducted in childhood ADHD [68], while children with mixed symptoms had more behavioral problems and emotional lability, the ADHD-I group showed more impairment in sustained attention and higher degree of brain white matter alterations.
Our findings should be interpreted with the acknowledgment of some strengths and limitations. It is important that all the patients had a valid ADHD diagnosis and were examined for potential comorbidities according to DSM-5 by experienced clinical professionals. These steps are crucial to get a more accurate picture and were often limitations in previous research [22]. It is important to note that the intelligence level is relatively high in our patient sample, while IQ scores for healthy controls was not available. The high average IQ score in patients suggests that the generalizability of our results is limited and the possibility of compensation strategies is also plausible, a feature that is both a strength and limitation of the study. The statistical approach was designed to control for multivariate effects. Unfortunately, contrary to recommendations, we did not have the opportunity to use Stop Signal Task (which measures response inhibition), because of language limitations at the time of data-acquisition, which is a limitation of the study. Although CANTAB has many advantages, among others, culture-independency, standardized, computerized measurement, assessment of several cognitive domains, it should be emphasized that it relates mainly to spatial-visual functions and we do not have information about other modalities. It is worth mentioning that some people with ADHD do not show any significant deficits compared to controls, that is why future studies should target the individual’s cognitive profile, rather than looking at differences from the average. Furthermore, a major challenge for the future can be to develop more ecologically valid test conditions that better model everyday life and the many stimuli we face while trying to perform efficiently. In addition, future work would benefit of including a predominantly hyperactive-impulsive subgroup to better understand the differences in terms of neurocognitive alterations between aADHD subtypes.
In summary, living with ADHD is often linked to individual suffering and decreased quality-of-life, difficulties in relationships, performance, realistic self-evaluation, moreover, without receiving diagnosis and treatment there is an increased risk of comorbid disorders, accidents, and last but not least, it results in high social costs [32]. Therefore, adequate and accessible screening possibilities, and cooperation with treatment can be crucial. The neuropsychological profile of aADHD is characterized by high heterogeneity, and the deficits of the symptom-based subgroups are different, which underscores the importance of individual-based treatment plans. Moreover, our results highlight the need for a holistic approach when assessing weaknesses and strengths in neurocognitive performance, including subjective and objective measures. Self-reported measures are important because they reflect the patients’ experience, while objective neuropsychological measures can be related more closely to functional impairments. Furthermore, the severity of neuropsychological alterations can predict the long term outcome including overall functioning and symptom severity [20, 21]. These aspects may also facilitate treatment-adherence, including pharmacological and non-pharmacological interventions.
Data availability
The data underlying this article are available on reasonable request.
References
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders, 5th edn. American Psychiatric Publishing, Inc, Washington
Faraone SV, Asherson P, Banaschewski T, Biederman J, Buitelaar JK, Ramos-Quiroga JA, Rohde LA, Sonuga-Barke EJ, Tannock R, Franke B (2015) Attention-deficit/hyperactivity disorder. Nat Rev Dis Primers 1:15020. https://doi.org/10.1038/nrdp.2015.20
Katzman MA, Bilkey TS, Chokka PR, Fallu A, Klassen LJ (2017) Adult ADHD and comorbid disorders: clinical implications of a dimensional approach. BMC Psychiatry 17(1):302. https://doi.org/10.1186/s12888-017-1463-3
Kittel-Schneider S et al (2022) Non-mental diseases associated with ADHD across the lifespan: Fidgety Philipp and Pippi Longstocking at risk of multimorbidity? Neurosci Biobehav Rev 132:1157–1180. https://doi.org/10.1016/j.neubiorev.2021.10.035
Faraone SV, Larsson H (2019) Genetics of attention deficit hyperactivity disorder. Mol Psychiatry 24(4):562–575. https://doi.org/10.1038/s41380-018-0070-0
Faraone SV, Biederman J (2005) What is the prevalence of adult ADHD? Results of a population screen of 966 adults. J Atten Disord 9(2):384–391. https://doi.org/10.1177/1087054705281478
Faraone SV, Sergeant J, Gillberg C, Biederman J (2003) The worldwide prevalence of ADHD: is it an American condition? World Psychiatry 2(2):104–113
Fayyad J et al (2017) The descriptive epidemiology of DSM-IV Adult ADHD in the World Health Organization World Mental Health Surveys. Atten Deficit Hyperact Disord 9(1):47–65. https://doi.org/10.1007/s12402-016-0208-3
Simon V, Czobor P, Bálint S, Mészáros A, Bitter I (2009) Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry 194(3):204–211. https://doi.org/10.1192/bjp.bp.107.048827
Anker E, Haavik J, Heir T (2020) Alcohol and drug use disorders in adult attention-deficit/hyperactivity disorder: prevalence and associations with attention-deficit/hyperactivity disorder symptom severity and emotional dysregulation. World J Psychiatry 10(9):202–211. https://doi.org/10.5498/wjp.v10.i9.202
Anker E, Ginsberg Y, Heir T (2021) Prevalence of criminal convictions in Norwegian adult ADHD outpatients and associations with ADHD symptom severity and emotional dysregulation. BMC Psychiatry 21(1):226. https://doi.org/10.1186/s12888-021-03223-0
Franke B, Michelini G, Asherson P et al (2018) Live fast, die young? A review on the developmental trajectories of ADHD across the lifespan. Eur Neuropsychopharmacol 28(10):1059–1088. https://doi.org/10.1016/j.euroneuro.2018.08.001
Hartman CA, Larsson H, Vos M, Bellato A, Libutzki B, Solberg BS, Chen Q, Du Rietz E, Mostert JC, Kittel-Schneider S, Cormand B, Ribasés M, Klungsøyr K, Haavik J, Dalsgaard S, Cortese S, Faraone SV, Reif A (2023) Anxiety, mood, and substance use disorders in adult men and women with and without attention-deficit/hyperactivity disorder: a substantive and methodological overview. Neurosci Biobehav Rev 151:105209. https://doi.org/10.1016/j.neubiorev.2023.105209
Sonuga-Barke EJ, Becker SP, Bölte S, Castellanos FX, Franke B, Newcorn JH, Nigg JT, Rohde LA, Simonoff E (2023) Annual Research Review: perspectives on progress in ADHD science—from characterization to cause. J Child Psychol Psychiatry 64(4):506–532. https://doi.org/10.1111/jcpp.13696
Asherson P, Buitelaar J, Faraone SV, Rohde LA (2016) Adult attention-deficit hyperactivity disorder: key conceptual issues. Lancet Psychiatry 3(6):568–578. https://doi.org/10.1016/S2215-0366(16)30032-3
Mostert JC, Onnink AMH, Klein M, Dammers J, Harneit A, Schulten T, van Hulzen KJE, Kan CC, Slaats-Willemse D, Buitelaar JK, Franke B, Hoogman M (2015) Cognitive heterogeneity in adult attention deficit/hyperactivity disorder: a systematic analysis of neuropsychological measurements. Eur Neuropsychopharmacol 25(11):2062–2074. https://doi.org/10.1016/j.euroneuro.2015.08.010
Barkley RA (1997) Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull 121(1):65–94. https://doi.org/10.1037/0033-2909.121.1.65
Sonuga-Barke EJ (2003) The dual pathway model of AD/HD: an elaboration of neuro-developmental characteristics. Neurosci Biobehav Rev 27(7):593–604. https://doi.org/10.1016/j.neubiorev.2003.08.005
Low AM, le Sommer J, Vangkilde S, Fagerlund B, Glenthøj B, Sonuga-Barke E, Habekost T, Jepsen JRM (2018) Delay aversion and executive functioning in adults with attention-deficit/hyperactivity disorder: before and after stimulant treatment. Int J Neuropsychopharmacol 21(11):997–1006. https://doi.org/10.1093/ijnp/pyy070
van Lieshout M, Luman M, Buitelaar J, Rommelse NN, Oosterlaan J (2013) Does neurocognitive functioning predict future or persistence of ADHD? A systematic review. Clin Psychol Rev 33(4):539–560. https://doi.org/10.1016/j.cpr.2013.02.003
van Lieshout M, Luman M, Twisk JW, Faraone SV, Heslenfeld DJ, Hartman CA, Hoekstra PJ, Franke B, Buitelaar JK, Rommelse NN, Oosterlaan J (2017) Neurocognitive predictors of ADHD outcome: a 6-year follow-up study. J Abnorm Child Psychol 45(2):261–272. https://doi.org/10.1007/s10802-016-0175-3
Onandia-Hinchado I, Pardo-Palenzuela N, Diaz-Orueta U (2021) Cognitive characterization of adult attention deficit hyperactivity disorder by domains: a systematic review. J Neural Transm (Vienna) 128(7):893–937. https://doi.org/10.1007/s00702-021-02302-6
Pollak Y, Shalit R, Aran A (2018) Risk taking and adult attention deficit/hyperactivity disorder: a gap between real life behavior and experimental decision making. Psychiatry Res 259:56–62. https://doi.org/10.1016/j.psychres.2017.10.012
Pollak Y, Dekkers TJ, Shoham R, Huizenga HM (2019) Risk-taking behavior in attention deficit/hyperactivity disorder (ADHD): a review of potential underlying mechanisms and of interventions. Curr Psychiatry Rep 21(5):33. https://doi.org/10.1007/s11920-019-1019-y
Robbins TW, James M, Owen AM, Sahakian BJ, Lawrence AD, McInnes L, Rabbitt PM (1998) A study of performance on tests from the CANTAB battery sensitive to frontal lobe dysfunction in a large sample of normal volunteers: implications for theories of executive functioning and cognitive aging. Cambridge Neuropsychological Test Automated Battery. J Int Neuropsychol Soc 4(5):474–490. https://doi.org/10.1017/s1355617798455073
Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P (1994) Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia 5(5):266–281. https://doi.org/10.1159/000106735
Fried R, DiSalvo M, Kelberman C, Biederman J (2021) Can the CANTAB identify adults with attention-deficit/hyperactivity disorder? A controlled study. Appl Neuropsychol Adult 28(3):318–327. https://doi.org/10.1080/23279095.2019.1633328
Nikolas MA, Nigg JT (2013) Neuropsychological performance and attention-deficit hyperactivity disorder subtypes and symptom dimensions. Neuropsychology 27(1):107–120. https://doi.org/10.1037/a0030685
Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF (2005) Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry 57(11):1336–1346. https://doi.org/10.1016/j.biopsych.2005.02.006
Dobson-Patterson R, O’Gorman JG, Chan RC, Shum DH (2016) ADHD subtypes and neuropsychological performance in an adult sample. Res Dev Disabil 55:55–63. https://doi.org/10.1016/j.ridd.2016.03.013
Phillips MS, Bing-Canar H, Shields AN, Cerny B, Chang F, Wisinger AM, Leib SI, Ovsiew GP, Resch ZJ, Jennette KJ, Soble JR (2023) Assessment of learning and memory impairments in adults with predominately inattentive versus combined presentation attention-deficit/hyperactivity disorder. Appl Neuropsychol Adult. https://doi.org/10.1080/23279095.2023.2169887
Fuermaier ABM, Tucha L, Koerts J, Aschenbrenner S, Kaunzinger I, Hauser J, Weisbrod M, Lange KW, Tucha O (2015) Cognitive impairment in adult ADHD–perspective matters! Neuropsychology 29(1):45–58. https://doi.org/10.1037/neu0000108
Balázs J, Bitter I, Hideg K, Vitrai J (1998) The Hungarian version of the MINI and MINI Plus). Psychiatr Hung 13(2):160–168
Balázs J, Bitter I (2000) Criterion-validity test of M.I.N.I Plus diagnostic questionnaire. Psychiatr Hung 15(2):134–144
Lecrubier Y, Sheehan DV, Weiller E, Amorim P, Bonora I, Sheehan KH, Janavs J, Dunbar GC (1997) The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. Eur Psychiatry 12(5):224–231. https://doi.org/10.1016/S0924-9338(97)83296-8
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998) The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59(Suppl 20):22–57
First MB, Gibbon M (2004) The structured clinical interview for DSM-IV axis I disorders (SCID-I) and the structured clinical interview for DSM-IV axis II disorders (SCID-II). In: Hilsenroth M, Segalaniel DL (eds) Comprehensive handbook of psychological assessment. Personality assessment, vol 2. Wiley, Hoboken, pp 134–143
Derogatis LR, Cleary PA (1977) Confirmation of the dimensional structure of the scl-90: a study in construct validation. J Clin Psychol 33(4):981–989. https://doi.org/10.1002/1097-4679(197710)33:4%3c981::AID-JCLP2270330412%3e3.0.CO;2-0
Derogatis LR, Cleary PA (1977) Factorial invariance across gender for the primary symptom dimensions of the SCL-90. Br J Soc Clin Psychol 16(4):347–356. https://doi.org/10.1111/j.2044-8260.1977.tb00241.x
Conners CK, Erhardt D, Sparrow EP (1999) Conners’ adult ADHD rating scales (CAARS): technical manual. Multi-Health Systems, New York
Balogh L, Kakuszi B, Papp S, Tombor L, Bitter I, Czobor P (2017) Neural correlates of error monitoring in adult attention deficit hyperactivity disorder after failed inhibition in an emotional Go/No-Go task. J Neuropsychiatry Clin Neurosci 29(4):326–333. https://doi.org/10.1176/appi.neuropsych.16100183
Bitter I, Mohr P, Balogh L, Látalová K, Kakuszi B, Stopková P, Zmeškalová-Jelenová D, Pulay A, Czobor P (2019) ADHD: a hidden comorbidity in adult psychiatric patients. Atten Deficit Hyperact Disord 11(1):83–89. https://doi.org/10.1007/s12402-019-00285-9
Papp S, Tombor L, Kakuszi B, Balogh L, Réthelyi JM, Bitter I, Czobor P (2020) Impaired early information processing in adult ADHD: a high-density ERP study. BMC Psychiatry 20(1):292. https://doi.org/10.1186/s12888-020-02706-w
Csukly G, Czobor P, Simon L, Takács B (2008) Basic emotions and psychological distress: association between recognition of facial expressions and Symptom Checklist-90 subscales. Compr Psychiatry 49(2):177–183. https://doi.org/10.1016/j.comppsych.2007.09.001
Unoka Z, Rózsa S, Kő N, Kállai J, Fábián Á, Simon L (2004) Validity and reliability of the SCL-90 in a Hungarian population sample. Psychiatr Hung 19:235–243
Wechsler D (1981) Wechsler adult intelligence scale-revised. Psychological Corporation, New York
Sahakian BJ, Morris RG, Evenden JL, Heald A, Levy R, Philpot M, Robbins TW (1988) A comparative study of visuospatial memory and learning in Alzheimer-type dementia and Parkinson’s disease. Brain 111(Pt 3):695–718. https://doi.org/10.1093/brain/111.3.695
Bartók E, Berecz R, Glaub T, Degrell I (2005) Cognitive functions in prepsychotic patients. Prog Neuropsychopharmacol Biol Psychiatry 29(4):621–625. https://doi.org/10.1016/j.pnpbp.2005.01.008
IBM Corp. (2013) IBM SPSS statistics for windows, version 22.0. IBM Corp., Armonk
SAS Institute (2016) The SAS system for windows (Release 9.4). SAS Institute, Cary
Jöreskog KG (1966) Testing a simple structure hypothesis in factor analysis. Psychometrika 31(2):165–178. https://doi.org/10.1007/BF02289505
Byrne BM, Shavelson RJ, Muthén B (1989) Testing for the equivalence of factor covariance and mean structures: the issue of partial measurement invariance. Psychol Bull 105(3):456–466. https://doi.org/10.1037/0033-2909.105.3.456
Jöreskog KG (1971) Simultaneous factor analysis in several populations. Psychometrika 36:409–426. https://doi.org/10.1007/BF02291366
Widaman KF, Reise SP (1997) Exploring the measurement invariance of psychological instruments: applications in the substance use domain. In: Bryant KJ, Windle ME, West SG (eds) The science of prevention: methodological advances from alcohol and substance abuse research. American Psychological Association, Washington, DC, pp 281–324
Shang CY, Lin HY, Gau SS (2021) The norepinephrine transporter gene modulates intrinsic brain activity, visual memory, and visual attention in children with attention-deficit/hyperactivity disorder. Mol Psychiatry 26(8):4026–4035. https://doi.org/10.1038/s41380-019-0545-7
Guo N, Koerts J, Tucha L, Fetter I, Biela C, König M, Bossert M, Diener C, Aschenbrenner S, Weisbrod M, Tucha O, Fuermaier ABM (2022) Stability of attention performance of adults with ADHD over time: evidence from repeated neuropsychological assessments in one-month intervals. Int J Environ Res Public Health 19(22):15234. https://doi.org/10.3390/ijerph192215234
Braet W, Johnson KA, Tobin CT, Acheson R, McDonnell C, Hawi Z, Barry E, Mulligan A, Gill M, Bellgrove MA, Robertson IH, Garavan H (2011) fMRI activation during response inhibition and error processing: the role of the DAT1 gene in typically developing adolescents and those diagnosed with ADHD. Neuropsychologia 49(7):1641–1650. https://doi.org/10.1016/j.neuropsychologia.2011.01.001
Skirrow C, McLoughlin G, Banaschewski T, Brandeis D, Kuntsi J, Asherson P (2015) Normalisation of frontal theta activity following methylphenidate treatment in adult attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol 25(1):85–94. https://doi.org/10.1016/j.euroneuro.2014.09.015
Bellgrove MA, Hawi Z, Kirley A, Gill M, Robertson IH (2005) Dissecting the attention deficit hyperactivity disorder (ADHD) phenotype: sustained attention, response variability and spatial attentional asymmetries in relation to dopamine transporter (DAT1) genotype. Neuropsychologia 43(13):1847–1857. https://doi.org/10.1016/j.neuropsychologia.2005.03.011
Kuc K, Bielecki M, Racicka-Pawlukiewicz E, Czerwinski MB, Cybulska-Klosowicz A (2020) The SLC6A3 gene polymorphism is related to the development of attentional functions but not to ADHD. Sci Rep 10(1):6176. https://doi.org/10.1038/s41598-020-63296-x
Fried R, Hirshfeld-Becker D, Petty C, Batchelder H, Biederman J (2015) How informative is the CANTAB to assess executive functioning in children with ADHD? A controlled study. J Atten Disord 19(6):468–475. https://doi.org/10.1177/1087054712457038
Mostert JC, Hoogman M, Onnink AMH, van Rooij D, von Rhein D, van Hulzen KJE, Dammers J, Kan CC, Buitelaar JK, Norris DG, Franke B (2018) Similar subgroups based on cognitive performance parse heterogeneity in adults with ADHD and healthy controls. J Atten Disord 22(3):281–292. https://doi.org/10.1177/1087054715602332
Haring L, Mõttus R, Koch K, Trei M, Maron E (2015) Factorial validity, measurement equivalence and cognitive performance of the Cambridge Neuropsychological Test Automated Battery (CANTAB) between patients with first-episode psychosis and healthy volunteers. Psychol Med 45(9):1919–1929. https://doi.org/10.1017/S0033291714003018
Smith PJ, Need AC, Cirulli ET, Chiba-Falek O, Attix DK (2013) A comparison of the Cambridge Automated Neuropsychological Test Battery (CANTAB) with “traditional” neuropsychological testing instruments. J Clin Exp Neuropsychol 35(3):319–328. https://doi.org/10.1080/13803395.2013.771618
Ceroni M, Rossi S, Zerboni G, Biglia E, Soldini E, Izzo A, Morellini L, Sacco L (2022) Attentive-executive functioning and compensatory strategies in adult ADHD: a retrospective case series study. Front Psychol 13:1015102. https://doi.org/10.3389/fpsyg.2022.1015102
Ullsperger M, Danielmeier C, Jocham G (2014) Neurophysiology of performance monitoring and adaptive behavior. Physiol Rev 94(1):35–79. https://doi.org/10.1152/physrev.00041.2012
Lin YJ, Gau SS (2020) Comparison of neuropsychological functioning between adults with early- and late-onset DSM-5 ADHD. J Atten Disord 24(1):29–40. https://doi.org/10.1177/1087054717730609
Wu ZM, Wang P, Liu L, Liu J, Cao XL, Sun L, Cao QJ, Yang L, Wang YF, Yang BR (2022) ADHD-inattentive versus ADHD-combined subtypes: a severity continuum or two distinct entities? A comprehensive analysis of clinical, cognitive and neuroimaging data. J Psychiatr Res 149:28–36. https://doi.org/10.1016/j.jpsychires.2022.02.012
Acknowledgements
The authors would like to thank Eszter Kenézlői, Katalin Vincze, Éva Magos, Zsuzsa Halmai, and Bettina Bajzát for their contributions in data collection, data curation and project administration. Funding by the Hungarian Brain Research Programme and the National Research Development and Innovation Office are gratefully acknowledged.
Funding
Open access funding provided by Semmelweis University. The research was funded by the Hungarian Brain Research Programme Grant 2017-1.2.1-NKP-2017-00002, and the National Research Development and Innovation Office Grants NKFI K129195 and TKP2021-EGA-25, all to JMR.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Somogyi, S., Kilencz, T., Szőcs, K. et al. Differential neurocognitive profiles in adult attention-deficit/hyperactivity disorder subtypes revealed by the Cambridge Neuropsychological Test Automated Battery. Eur Arch Psychiatry Clin Neurosci (2023). https://doi.org/10.1007/s00406-023-01702-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00406-023-01702-x