Abstract
As assessed by numerous neuropsychological tasks, individuals with autism spectrum disorder (ASD) and schizophrenia spectrum disorders (SSDs) have similar impairments related to executive functions (EFs). The neuropsychological profile of these two conditions was examined using the three-component EFs’ framework of Miyake and Friedman (Cogn Psychol 41(1):49-100, 2000). This approach assesses Inhibition (suppression of unwanted and irrelevant information/responses), Updating (use and control of contents of working memory), and Shifting (disengagement between activities or mental tasks) using nine different tasks. In line with previous research, we expected greater performance deficits in ASD in all three components compared to SSD, as well as faster responses for the SSD group. A self-paced task format allowed us to examine whether unlimited time given for a task would lead to better performance. The sample was constituted by the control group (N = 25), ASD group (N = 24), and SSD group (N = 12). Groups did not differ on Inhibition performance. In Updating, individuals with SSD performed poorer than the other groups. As for Shifting, both groups demonstrated poorer performance compared to controls, with the SSD group presenting the greatest difficulties. In terms of reaction time (RT), SSD participants’ RT were the slowest on Inhibition and Shifting tasks. There was a positive correlation between performance and time spent on Inhibition and Shifting only for the SSD group, which demonstrates that their performance improves when there are no time constraints. Our work provides a better understanding of spared and impaired EFs, which could be useful for designing strategies aimed at improving specific EFs in each group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism spectrum disorder (ASD) and schizophrenia spectrum disorder (SSD) are two conditions recognized by classification systems, such as the diagnostic and statistical manual of mental disorders—DSM-5 [1], as two distinct entities. In ASD, core symptoms include severe difficulties in social communication and restricted or repetitive patterns of behavior and interests. These symptoms usually manifest themselves in early childhood and are maintained throughout the individual’s life [1]. In SSD, however, symptoms usually appear in late adolescence or, more frequently, early adulthood. The symptoms of SSD are grouped into two categories: positive symptoms and negative symptoms [1]. Positive symptoms include hallucinations and/or delusions, whereas negative symptoms refer to the absence or reduction of behaviors such as emotional expression or the scarcity of communicative gestures.
In general, a person who displays typical clinical psychotic symptoms, such as hallucinations and delusions, would be diagnosed with SSD. However, many of the symptoms presented by people with ASD can be mistaken for SSD symptoms. For example, difficulties in social communication and social-emotional reciprocity, sensory disturbances, and rigidity in thinking are features shared by the two disorders [2,3,4]. Thus, the overlap in symptoms can lead to diagnostic confusion [4]. On a biological level, the two conditions share similar genetic modifications in DNA sequence (copy-number variations, CNV) and specific rare alleles [5]. On the neurological level, some magnetic resonance imaging (MRI) studies in ASD and SSD have shown similar alterations in the morphology of the posterior lobe of the cerebellum [6], and others demonstrated the same type of grey matter volume abnormalities in regions of the frontal and parietal lobes for the two conditions [7,8,9]. These neurobiological similarities, added to the similarities in behavioral symptomatology, generally make differential diagnosis difficult, especially in adulthood and if the individual did not receive a diagnosis of ASD in childhood. The present study aims to advance research on similarities and differences in the neuropsychological profiles of the two conditions. The long-term goal is to facilitate differential diagnosis and treatment strategies.
Executive Functions (EFs) are one of the aspects in which ASD and SSD present strikingly similar characteristics, which may influence the difficulties in differential diagnosis [10, 11]. EFs are known to be fundamental for learning, academic performance, mental health, adaptive behaviors [12, 13], and goal-directed behaviors [14]. Past research has demonstrated that the poor outcomes in personal, academic, vocational, or everyday functioning displayed by individuals with ASD or SSD have, indeed, been attributed to impairments in EF abilities [9]. Although scarce, the existing evidence suggests that, while in ASD the EF difficulties persist through adulthood [15], in SSD, there is a visible decline in EF in aging and after psychotic episodes [16].
Despite the lack of consensus on how to best assess EF, researchers agree that it is not a unitary domain but rather encapsulates a series of domains and abilities. Although there are different EF models, many of them suggest Inhibition, Updating and Shifting [12, 17] as the core components of EFs. This three-component conceptualization was first introduced by Miyake et al. [17] as the Unity and Diversity theory of executive functioning. The Unity and Diversity framework is a well-recognized and science-based assessment approach, wherein each EF component is measured using three different tasks. Miyake et al. [17] suggested that EF components work, both independently and interactively with one another. A confirmatory factor analysis (CFA) demonstrated that these three components are statistically separable into clusters, but since they are not perfectly correlated, they could still share a great portion of features between components [18]. Thus, the novelty of our study is to assess ASD and SSD EFs using Miyake and Friedman’s framework, which offers a task-based approach [17,18,19,20] that covers the core components of EFs found to be affected in the two disorders. We describe each component as follows.
Inhibition
Inhibition is fundamental when it comes to suppressing unwanted responses to minimize the processing of irrelevant information and for selecting useful or relevant information to respond appropriately to a given situation [21], or to successfully complete a task [19, 22]. To date, literature on Inhibition in adults with ASD shows mixed results, with some studies showing spared functioning of this component [23], while others find impairments [24]. For example, in a study with adults with ASD, deficits were observed using a random-motor-generation task where participants were asked to inhibit motor-prepotent responses [25]. Individuals with SSD were also found to make more errors in inhibiting motor responses in tasks like the Stroop [21]. Furthermore, Ettinger et al., [21] showed that individuals with SSD make more mistakes when the task requires them to, not only inhibit a prepotent response, but also to produce an alternative response (e.g., in Antisaccade and Stroop tasks). However, in tasks where these individuals were required to only suppress an unwanted response (e.g., in the Stop-Signal task), their performance was intact.
Updating
This component involves the ability to encode information in long-term memory, to retrieve it, and to subsequently use that information [12]. Moreover, it refers to the ability to monitor and control the contents of working memory and facilitates the access to relevant information [22]. In this fashion, Updating is very much linked to working memory capacity. Indeed, research suggests that for correct functioning, Updating requires working memory to incorporate new information of ongoing, planned behaviors and actions [12]. In their review, Gold et al. [26] found mixed results in SSD whereby some studies reported deficits and others report a spared Updating capacity. A great deal of research conducted in children and adolescents with ASD showed deficits in complex tasks that required both management of previous stored information and maintenance of immediate information, such as keeping track of stimuli. Furthermore, it seems that when the memory load is higher (e.g., keeping track of more than two objects), participants with ASD exhibit poorer performance [25]. A study assessing Updating in ASD and SSD revealed that both disorders have a lower working memory capacity as compared to healthy controls [11].
Shifting
Shifting ability allows the individual to disengage from one activity or mental set to another. Also, it involves switching flexibly from one thought, action, activity, or situation to another [15, 22, 27]. Arguably, problems in Shifting can account for some repetitive and restrictive behaviors observed in ASD [28]. For example, Albein-Urios et al. [28] argued that 69% of young adults with ASD showed important difficulties in performing Shifting tasks as expressed by the Shift index of the BRIEF informant-report [29]. Sarro et al. [15] further suggested that Shifting difficulties are reflected in the fact that people with ASD persevere in their responses even after receiving corrective feedback on their performance. Similar impairments have been found in Shifting in SSD using neuropsychological tests like the Trail Making Test, showcasing similar perseverance and rigidity [30], as these individuals continued with the same response style after receiving negative feedback on their performance.
The Miyake and Friedman three-component framework [17, 31] is appropriate for our study given that it has been used in many clinical groups [8, 13], including SSD [22], and across different age groups (children [32] and adults [17, 19]). To our knowledge, our study is the first one that attempts to understand the pattern of shared and independent deficits in EFs in both ASD and SSD. The motivation behind carrying out a comparative study is twofold. First, it is highly relevant to be able to make a better differentiation of the two disorders, because the similarities in symptomatology can lead to misdiagnosis. Despite the fact that comparative studies that look for differences in underlying mechanisms that contribute to symptoms can be useful for correct diagnosis, the research is rather scarce. Thus, the present study responds to this scarcity of research. Second, the misdiagnosis can lead to inadequate treatment. Thus, in a long run, differentiating between spared and impaired aspects of EF in each disorder can improve treatment in terms of designing more personalized intervention programs.
Our work is the first to use a computerized Spanish language adaptation of Miyake and Friedman’s [17, 31] task-based assessment to compare individuals with ASD and SSD. Specifically, we were interested in examining performance accuracy and the average reaction-time (RT). Although the nature of our study is exploratory, a previous study comparing the performance of ASD and SSD on some neuropsychological tasks found that both groups showed a lower performance in Inhibition-, Updating-, and Shifting-related tasks [11]. However, it is noteworthy that the results published so far are mixed, possibly due to methodological issues (small sample sizes, variety of EF tasks used, etc.). Also, to be able to draw firmer conclusions about the status of EF similarities and differences, we need to directly compare SSD and ASD individuals’ performance on EF tasks. In line with past research, we expected to observe deficits in performance across all three-core EF components compared to the control group, but our predictions about the pattern of results are rather exploratory in nature. That is, we are interested in looking for specific differences in the pattern of strengths and weaknesses on performance scores in the two clinical groups. In terms of RT and bearing in mind previous findings, we expected to find faster responses in the SSD group compared to the ASD group [33]. We used a self-paced task format which allowed us to examine the relationship between time spent on a given task and performance accuracy. Research emphasizes the importance of considering RT because of its relevance when assessing EFs [33]. For example, a study which compared EF in individuals with ASD and ADHD found their performance to improve significantly in tasks that had no time limit [34]. Therefore, we expected to see a positive correlation whereby spending more time on a task would allow participants in our study to reach greater accuracy in their performance.
Materials and methods
Participants
All participants from this study were assessed with the Wechsler Adult Intelligence Scale-IV (WAIS-IV) [35]. The participants who scored below the cut-off point of ≥ 70 on the IQ-Full-Scale of the WAIS-IV were excluded from the study. Each group is described below.
ASD group. Twenty-four participants with ASD participated in the study. All participants met the IQ inclusion criteria. All participants were diagnosed with ASD prior the study; however, we also confirmed the ASD diagnosis with the Autism Diagnostic Observation Schedule (ADOS-2) (Modules 3–4) [36]. The reason behind confirming the diagnosis is that the majority of the participants had a diagnosis at an early age or did not have updated psychological records. Thus, to have a reliable characterization of the sample, we decided to confirm the clinical diagnosis using the ADOS-2. We were unable to confirm the diagnosis of two participants due to time constraints. However, since these participants had a previous official ASD diagnosis, we did not consider it should be of concern from a methodological perspective. Also, to assess autistic traits in all groups, we administered the Autism Spectrum Quotient Short Form Spanish version (AQ-S) [37]. The cut-off point for autistic traits is > 63 (see Table 1).
SSD group. Fifteen participants with a diagnosis of SSD participated in the study. According to the information provided by their psychiatrist, they had no history of substance abuse in the 5 years prior to the study (e.g., use of alcohol, cannabis, hallucinogens, or opioids). To participate, no acute psychotic symptoms could be present at the time of the assessment, as determined by the Positive and Negative Syndrome Scale (PANSS) Spanish version [38, 39] (see Table 1). To assess autism co-occurrence, the ADOS-2 was administered. Finally, three participants were excluded from the study as they scored below IQ-Full-Scale cut-off point.
Typical Development Control group (TDC). Twenty-five participants were recruited from the general public and student population from the University of Salamanca. All participants met the IQ inclusion criteria. The exclusion criterion for this group was a score above the cut-off point on the AQ-S. No participants were excluded.
A clinical questionnaire pertaining the use of medication, previous medical history, and other mental health problems was obtained from all participants. In the ASD group, one participant reported having epilepsy and four participants reported taking medication. As for the SSD group, antipsychotic medication doses were within the guidelines and doses recommended by Spanish drug regulators in all cases.
Procedures
Before testing, informed consent for adult participants and parental consent for underage participants were collected. The study was approved by the Bioethical Committee of the Universidad de Salamanca. The testing was administered individually in two or three sessions, each with a maximum duration of 60–70 min. Sessions were conducted by a trained researcher.
Neuropsychological tasks
We followed Miyake et al.’s [17] and Friedman et al.’s [31] procedures to assess EFs. The tasks were computerized using OpenSesame [40], a Python-based software. Tasks were administered in a MacBook Pro 13". As with the original study, Updating was examined with Keep-Track, Letter-Memory and Spatial 2-Back task. Shifting was assessed with Number-Letter, Color-Shape, and Category-Switch task. Finally, Inhibition was examined with Antisaccade, Stop-Signal, and the Stroop task. Specific information about the details and design of each task can be found on the electronic Supplementary Information document. For each of the nine tasks administered, we obtained individual scores that were later computed into an overall domain score for each EF component. For Inhibition, we calculated the Hit-Rate Performance score (HR-P, reflected by the total accurate responses) and the mean RT in milliseconds (MS). For the Updating component, we obtained the HR-P score, but no RTs were analyzed as in the original design [17]. As for the Shifting component, three different scores were obtained: HR-P, mean RT, and the Switch-Cost (SC), which was the average time participants took switching between tasks.
Analysis
Analyses were conducted using SPSS 26.0 [41] and graphics were created using R [42]. We decided to run Kruskal–Wallis H test to examine group differences as well as post hoc analysis for all demographic characteristics. For the individual EF tasks, assumptions for conducting a parametric test were not met; therefore, we decided to run Kruskal–Wallis H test to determine group differences in the HR-P, RT, and SC across all tasks (see Table 2), as well as for the component total scores. Distributions of these variables were dissimilar for all groups, as assessed by visual inspection of a boxplot. Subsequently, pairwise group comparisons were performed using Dunn’s [43] procedure with a Bonferroni correction for multiple comparisons. Adjusted p values were reported with significance-level set at < 0.05.
Furthermore, we ran a Spearman’s correlation test to assess the relationship between accurate performance in the Inhibition and Shifting components and the RT from each group. Analyses showed that there were no outliers in our data and that the relationship found was linear with both variables normally distributed as assessed by the Shapiro–Wilk test (p > 0.05).
Results
Participants’ characteristics
Descriptive statistics of the participants’ characteristics are summarized in Table 1. We also reported differences between groups on some of those characteristics, as well as psychopharmacological use in Table 1.
Tasks performance and RT analysis
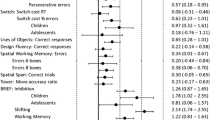
The descriptive statistics of group performance in all the tasks are depicted in Table 2. One participant from the ASD group reported being colorblind, which made him unable to perform one task that relied on colors. Also, not every participant in the SSD group could complete all the tasks, mainly because they could not retain the instructions during the practice trials, and those tasks were not completed. The neuropsychological profile obtained in the tasks is shown in Fig. 1A, B and C. We plotted our results using standardized mean differences (SMD).
SMD standard mean difference, HR-P hit-rate performance, RT reaction-time, ASD autism spectrum disorder, SSD schizophrenia spectrum disorders, TDC typical developmental controls, SC switch-cost. A Inhibition tasks profile by the groups. B Updating tasks’ profile by the groups. C Shifting tasks’ profile by the groups. Group differences are described by the group effect size as small, medium, or large
Component performance and RT analysis
Inhibition. For this component, there were no significant group differences between the groups in their performance H(2) = 5.01, p = 0.08, η2 = 0.13, the group effect size was medium. As for the RT obtained in Inhibition, we found a significant group difference, H(2) = 26.70, p < 0.05, η2 = 0.33, and the group effect size was large. Post hoc analyses showed differences between the TDC and ASD and the TDC and SSD group. That is, SSD participants’ RT was the slowest (M = 973.06MS, SD = 214.74), followed by the ASD group (M = 782.87MS, SD = 262.51) and the TDC group (M = 555.18MS, SD = 100.20).
Updating. For this component, we found statistically significant group differences in performance, H(2) = 11.68, p < 0.05, η2 = 0.15, and effect size was large. Post hoc analysis only showed significant group differences between the TDC and SSD group, whereby participants with SSD performed poorer (M = 0.95, SD = 0.18) than the ASD (M = 1.12, SD = 0.28) and than TDC group (M = 1.23, SD = 0.17).
Shifting. Results indicated significant group differences in the performance of Shifting H(2) = 15.87, p < 0.05, η2 = 0.22, and the effect size was large. Post hoc showed a significant group difference in the performance between the TDC and ASD group and between the TDC and SSD group. Both SSD (M = 0.93, SD = 0.31) and ASD group (M = 1.13, SD = 0.32) performance accuracy was significantly lower than the TDC group (M = 1.34, SD = 0.12). As for the RT in Shifting, we found significant group differences H(2) = 7.26, p < 0.05, η2 = 0.15, with a large-effect size. Here, the SSD (M = 2008.13MS, SD = 867.80) and ASD (M = 1923.80MS, SD = 890.44) groups obtained the slowest responses compared to the TDC group (M = 1405.78MS, SD = 352.560). As for the Switch-Cost in Shifting, we did not find significant group differences, H(2) = 5.57, p = 0.06, η2 = 0.11 (see Fig. 2, A and B, for the average scores obtained by the groups). For visual representation of the neuropsychological profile in executive functioning by groups, see Fig. 3.
HR-P hit-rate performance, EFs executive functions, RT reaction-time, ASD autism spectrum disorder, SSD schizophrenia spectrum disorders, TDC typical developmental controls, SC switch-cost. A Mean HR-P scores in the three-core components of EFs by the groups. B Mean RT scores obtained in Inhibition and Shifting and the mean SC in Shifting by the groups
SMD standard mean difference, HR-P hit-rate performance, RT reaction-time, ASD Autism spectrum disorder, SSD Schizophrenia spectrum disorders, TDC Typical developmental controls, SC Switch-cost. Executive function profile in each core domain by the groups. Group differences are described by the group effect size as small, medium, or large
Relationship between RT and performance accuracy
We found a strong positive correlation between the performance in Inhibition and time spent in the tasks (rs = 0.58, p < 0.05) in the SSD group. The correlation was not significant for the TDC (rs = 0.15, p = 0.49) and ASD groups (rs = − 0.15, p = 0.48). Similar results were found in Shifting performance score and the RT, where we found a strong positive correlation in the SSD group (rs = 0.87, p < 0.05), but not the TDC (rs = − 0.35, p = 0.08) and ASD groups (rs = − 0.15, p = 0.49). Finally, the results demonstrated that the SSD group had the slowest RTs in Inhibition and Shifting.
Discussion
The goal of this study was to examine the executive function profile of adults with ASD and SSD using, for the first time in the literature, a computerized task-based approach [17, 18]. Overall, we found that executive functioning difficulties were more pronounced in SSD than the ASD group. Also, contrary to what we expected, we found that the ASD group showed faster reaction times across the tasks compared to the SSD group.
When the Inhibition component was assessed, we found that all groups performed equally well, suggesting that the inhibitory mechanism was not altered in either of the groups. These findings show that, as assessed by tasks that target the suppression of irrelevant information or distractors, both ASD and SSD group’s performance was comparable to TDCs. However, individuals with SSD and ASD had slower RTs than controls, which indicates that they required significantly more time to complete these tasks as compared to the control group. When we looked at the relationship between RT and performance, only the SSD group showed the beneficial effect of having unlimited time—that is, the more time they spent on the task, the better their performance.
In terms of the Updating component, we found that individuals with ASD had comparable levels of performance to controls, contrary to what we predicted. Previous research in children and adolescents with ASD showed deficits in many aspects of the Updating component, such as problems with planning and monitoring actions; retrieving information from long-term memory; or updating ongoing activity [12, 22]. Given the results in our adult sample of individuals with ASD, it is plausible to think that this component of EF, while affected in childhood, does not remain impaired in adulthood [44]. As predicted, however, we observed poorer performance in SSD in Updating tasks compared to TDC performance. This finding is in line with previous studies in SSD that suggest great difficulties in Updating [16]. Note that reaction-time was not measured here.
With regards to the performance in the Shifting component, a clear pattern of difficulties in both individuals with ASD and with SSD was found, whereby they had significant difficulties in switching between activities accurately. Likewise, a different pattern of results from the TDC group was observed in terms of RT, in which the clinical groups took longer to complete the Shifting tasks. However, unlike with the inhibition tasks described above, more time spent on the task did not yield better performance levels in the ASD group. However, in the SSD group, there was a significant positive correlation between time and performance. A plausible explanation for this speed–accuracy trade-off in SSD is that participants with this disorder were older than the other comparison groups and slowing of reaction times with age is a common observation [45]. Notwithstanding, unlimited time did not bring the performance of individuals with SSD to typical levels observed in the control group. Finally, we did not find group differences in the Switch-Cost scores. This means that both groups were switching between tasks at a similar pace as the TDC group. It is worth noting, however, that although no significant differences were found between groups, some SSD participants did not complete parts of the tasks as they were unable to retain the instructions. This can be considered indicative of EF deficits whereby SSD individuals have problems in retaining information for the purpose of carrying out an ongoing task.
The outcomes from our study have several clinical implications. Assessing the strengths and weaknesses of EFs in these clinical groups may help us to establish not only the status of those aspects of the EF that are problematic, but also reinforce the use of those that are spared. For example, both individuals with SSD and ASD need support in tasks that involve Updating and Shifting skills, but not necessarily Inhibition skills. In terms of self-paced formats of activities, the outcomes of our study suggest that reducing time pressure is particularly beneficial for individuals with SSD, a finding that could be taken into account when planning vocational or occupational interventions. That is, a student with SSD could perform better in assignments by being given more time, while a professional with SSD could be provided with extended deadlines to aid their productivity. These specific interventions might influence the prognosis of achieving a successful adult life for individuals living with these disorders and essentially impact their quality of life, independence, and their ability to adapt to different day-to-day situations.
As mentioned earlier, a vast amount of research focuses on early development, and still little is known about EF abilities in later years. While Updating and Inhibition are typically impaired in childhood, they seem to be spared in adults with ASD. Some tentative accounts have been offered to explain the developmental trends in cognitive performance. A few studies in adults with ASD indicate that while certain EFs in autism are affected in early years, they improve with aging [46, 47]. Cognition and aging have received some attention in recent years. For example, Oberman and Pascual-Leone [48] found that older adults with autism do not present the same cognitive decline as older adults with early stages of Alzheimer Disease. This is particularly interesting, given that evidence shows memory and EF difficulties in children and adults with ASD. Oberman and Pascual-Leone [48] have explained this trend, suggesting that brain hyperplasticity in autism leads to brain underconnectivity in children and younger adults with ASD and contributes to cognitive impairments. However, during older age, it actually protects them from naturally occurring hypoplasticity in healthy aging (see literature on ASD [48, 49]). Also, the safeguard hypothesis [47] suggests that on a behavioral level, older adults with autism acquire, through life experiences, some compensatory strategies that help them to cope with their difficulties. Future longitudinal studies, or even cross-sectional studies with different age groups, could help us to ascertain the developmental trajectory of EFs in ASD and closely study the dynamic changes that seem to be occurring in EFs in this clinical group. On the other hand, the progression of cognitive trajectory in SSD is different to the one observed in ASD as there seems to be a decline, rather than an improvement in cognitive performance [50, 51].
Limitations and future research
Given small sample sizes and given that this was an opportunity sample, we could not match the groups on age, making our outcomes hard to generalize for the SSD group. Therefore, we believe that future research should try to recruit larger samples and compare the groups considering age as a covariate, as age has been associated with poorer performance and slower responses in older individuals with schizophrenia [45]. Nevertheless, we should note that even though our sample size was small, we found medium-to-large-effect sizes when assessing group differences in performance across our study.
A recent work from Yon-Hernández et al. [52] found that ASD individuals self-report more difficulties than individuals with SSD related to both EFs and adaptive behaviors in everyday life situations. These results differ from the findings of the current study, where we noted relatively more EF problems in SSD than ASD population. To verify the extent to which EF deficits have an impact on these individuals' everyday functioning and ability to adapt in life, future research should focus more on combining both types of assessment, i.e., neuropsychological, and more ecologically valid evaluations of EF.
Research in children with ASD has associated difficulties in EFs with perseverative responses, stereotyped behaviors, and difficulties at modulating motor acts [44]. As these are shared symptoms in both autism and schizophrenia, future research should directly study the relationship between these behavioral manifestations and the core components of EFs. Also, there is some overlap in problems in social interaction in both disorders [53, 54]. Thus, it would be of interest to determine whether difficulties in interacting with others are related to same (e.g., Shifting) or different (e.g., Updating) deficits in EF in these clinical populations.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders. American Psychiatric Association, Virginia
Fitzgerald M (2012) Schizophrenia and autism/asperger’s syndrome: overlap and difference. Clin Neuropsychiatry 9(4):171–176
Haigh SM, Eack SM, Keller T, Minshew NJ, Behrmann M (2019) White matter structure in schizophrenia and autism: Abnormal diffusion across the brain in schizophrenia. Neuropsychologia. 135.
Trevisan DA, Foss-Feig JH, Naples AJ, Srihari V, Anticevic A, McPartland JC (2020) Autism spectrum disorder and schizophrenia are better differentiated by positive symptoms than negative symptoms. Front Psych 11:11
Lionel AC, Vaags AK, Sato D, Gazzellone MJ, Mitchell EB, Chen HY et al (2013) Rare exonic deletions implicate the synaptic organizer Gephyrin (GPHN) in risk for autism, schizophrenia and seizures. Hum Mol Genet 22(10):2055–2066
Morimoto C, Nakamura Y, Kuwabara H, Abe O, Kasai K, Yamasue H et al (2021) Unique morphometric features of the cerebellum and cerebellocerebral structural correlation between autism spectrum disorder and schizophrenia. Biol Psychiatry Global Open Sci. https://doi.org/10.1016/j.bpsgos.2021.05.010
Cheung C, Yu K, Fung G, Leung M, Wong C, Li Q et al (2010) Autistic disorders and schizophrenia: related or remote? An anatomical likelihood estimation. PLoS ONE 5(8):e12233
Glisky EL, Alexander GE, Hou M, Kawa K, Woolverton CB, Zigman EK et al (2021) Differences between young and older adults in unity and diversity of executive functions. Aging, Neuropsychol Cogn 28(6):829–854. https://doi.org/10.1080/13825585.2020.1830936
Mazza M, Pino MC, Keller R, Vagnetti R, Attanasio M, Filocamo A et al (2021) Qualitative differences in attribution of mental states to other people in autism and schizophrenia: what are the tools for differential diagnosis? J Autism Dev Dis. https://doi.org/10.1007/s10803-021-05035-3
Sha Z, Schijven D, Francks C (2021) Patterns of brain asymmetry associated with polygenic risks for autism and schizophrenia implicate language and executive functions but not brain masculinization. Mol Psychiatry. https://doi.org/10.1038/s41380-021-01204-z
Marinopoulou M, Lugnegård T, Hallerbäck MU, Gillberg C, Billstedt E (2016) Asperger syndrome and schizophrenia: a comparative neuropsychological study. J Autism Dev Dis 46(7):2292–2304
Diamond A (2013) Executive functions. Ann Rev Psychol. 64(1):135–168
Hartung J, Engelhardt LE, Thibodeaux ML, Harden KP, Tucker-Drob EM (2020) Developmental transformations in the structure of executive functions. J Exp Child Psychol 189:104681. https://doi.org/10.1016/j.jecp.2019.104681
He L, Zhuang K, Chen Q, Wei D, Chen X, Fan J et al (2021) Unity and diversity of neural representation in executive functions. J Exp Psychol Gen 150(11):2193–2207
di Sarro R, di Santantonio A, Desideri L, Varrucciu N (2021) Profiling planning skills and cognitive flexibility of adults with autism spectrum disorders: Preliminary results from an exploratory service-based study. Int J Dev Disabil. https://doi.org/10.1080/20473869.2020.1871311
Muralidharan A, Finch A, Bowie CR, Harvey PD (2020) Older versus middle-aged adults with schizophrenia: Executive functioning and community outcomes. Schizophrenia Res 216:547–549
Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD (2000) The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cogn Psychol 41(1):49–100
Miyake A, Friedman NP (2012) The Nature and Organization of Individual Differences in Executive Functions. Curr Dir Psychol Sci 21(1):8–14
Friedman NP, Miyake A (2017) Unity and diversity of executive functions: individual differences as a window on cognitive structure. Cortex 86:186–204
Miyake A, Emerson MJ, Padilla F, Ahn J (2004) Inner speech as a retrieval aid for task goals: the effects of cue type and articulatory suppression in the random task cuing paradigm. Acta Psychol 115(2–3):123–142
Ettinger U, Aichert DS, Wöstmann N, Dehning S, Riedel M, Kumari V (2018) Response inhibition and interference control: effects of schizophrenia, genetic risk, and schizotypy. J Neuropsychol 12(3):484–510
Haywood D, Baughman FD (2021) Multidimensionality in executive function profiles in schizophrenia: a computational approach using the wisconsin card sorting task. Comput Brain Behav. 4(4):381–391
Hlavatá P, Kašpárek T, Linhartová P, Ošlejšková H, Bareš M (2018) Autism, impulsivity and inhibition a review of the literature. Basal Ganglia. 14:44
May KE, Kana RK (2020) Frontoparietal network in executive functioning in autism spectrum disorder. Autism Res 13(10):1762–1777
Weiss EM, Gschaidbauer B, Kaufmann L, Fink A, Schulter G, Mittenecker E et al (2017) Age-related differences in inhibitory control and memory updating in boys with Asperger syndrome. Eur Arch Psychiatry Clin Neurosci 267(7):651–659
Gold JM, Robinson B, Leonard CJ, Hahn B, Chen S, McMahon RP et al (2018) Selective attention, working memory, and executive function as potential independent sources of cognitive dysfunction in schizophrenia. Schizophr Bull 44(6):1227–1234
Hill EL, Bird CM (2006) Executive processes in Asperger syndrome: Patterns of performance in a multiple case series. Neuropsychologia 44(14):2822–2835
Albein-Urios N, Youssef GJ, Kirkovski M, Enticott PG (2018) Autism spectrum traits linked with reduced performance on self-report behavioural measures of cognitive flexibility. J Autism Dev Dis 48(7):2506–2515
Gioia GA, Isquith PK, Guy SC, Kenworthy L (2015) Behavior rating inventory of executive function. 2nd edn. Lutz, (FL); Psychological Assessment Resources, Inc
Mittal P, Mehta S, Solanki R, Swami M (2013) A comparative study of cognitive flexibility among first episode and multi-episode young schizophrenia patients. German J Psychiatry 16:130–136
Friedman NP, Miyake A, Young SE, DeFries JC, Corley RP, Hewitt JK (2008) Individual differences in executive functions are almost entirely genetic in origin. J Exp Psychol Gen 137(2):201
Miller MR, Giesbrecht GF, Müller U, McInerney RJ, Kerns KA (2012) A latent variable approach to determining the structure of executive function in preschool children. J Cogn Dev 13(3):395–423
de Boer M, Spek AA, Lobbestael J (2014) Comparing cognitive functioning in schizophrenia and autism using WAIS-III. Res Autism Spectr Dis 8(7):737–745
Karalunas SL, Huang-Pollock CL (2013) Integrating impairments in reaction time and executive function using a diffusion model framework. J Abnorm Child Psychol 41(5):837–850
Wechsler D (2012) WAIS-IV: escala de inteligencia de wechsler para adultos IV. Pearson, Madrid
Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop SL (2015) ADOS-2. Autism Diagnostic Observation Schedule-2. Madrid (ES): TEA Ediciones
Lugo-Marín J, Díez-Villoria E, Magán-Maganto M, Pérez-Méndez L, Alviani M, de la Fuente-Portero JA et al (2019) Spanish validation of the autism quotient short form questionnaire for adults with autism spectrum disorder. J Autism Dev Dis 49(11):4375
Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bull 13(2):26
Peralta V, Cuesta MJ (1994) Psychometric properties of the positive and negative syndrome scale (PANSS) in schizophrenia. Psychiatry Res 53(1):31–40
Mathôt S, Schreij D, Theeuwes J (2012) opensesame: an open-source, graphical experiment builder for the social sciences. Behav Res Methods 44(2):314
Corp IBM (2019) IBM SPSS Statistics for Windows, Version 26,0. BM Corp, Armonk
R Core Team R (2021) A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria
Dunn OJ (1964) Multiple comparisons using rank sums. Technometrics 6:241–252
Hill EL (2004) Executive dysfunction in autism. Trends Cogn Sci 8(1):26–32
Lee J, Green MF, Nuechterlein KH, Swerdlow NR, Greenwood TA et al (2020) The effects of age and sex on cognitive impairment in schizophrenia: findings from the consortium on the genetics of schizophrenia (COGS) study. PLoS ONE 15(5):e0232855. https://doi.org/10.1371/journal.pone.0232855
Davids RCD, Groen Y, Berg IJ, Tucha OM, van Balkom IDC (2016) Executive functions in older adults with autism spectrum disorder: objective performance and subjective complaints. J Autism Dev Dis 46(9):2859–2873
Geurts HM, Vissers ME (2012) Elderly with autism: executive functions and memory. J Autism Dev Dis 42(5):665–675
Oberman LM, Pascual-Leone A (2014) Hyperplasticity in autism spectrum disorder confers protection from alzheimer’s disease. Med Hypotheses 83(3):337–342
Wilson JF, Lodhia V, Courtney DP, Kirk IJ, Hamm JP (2017) Evidence of hyper-plasticity in adults with autism spectrum disorder. Res Autism Spectr Dis 43–44:40–52
Keefe RSE, Harvey PD (2012) Cognitive impairment in schizophrenia. Springe, Berlin, pp 11–37
Bhattacharya K (2015) Cognitive function in schizophrenia: a review. J Psychiatry. https://doi.org/10.4172/Psychiatry.1000187
Yon-Hernández JA, Wojcik DZ, García-García L, Franco-Martín MA, Canal-Bedia R (2022) Differences in daily life executive functioning between people with autism and people with schizophrenia. J Autism Dev Dis. https://doi.org/10.1007/s10803-022-05547-6
Spek AA, Wouters SGM (2010) Autism and schizophrenia in high functioning adults: behavioral differences and overlap. Res Autism Spectr Dis 4(4):709–717
Stone WS, Iguchi L (2011) Do apparent overlaps between schizophrenia and autistic spectrum disorders reflect superficial similarities or etiological commonalities? Am Chin J Med Sci. 4(3):124–133
Acknowledgements
The authors want to thank the participants for their contribution to this study. We acknowledge María de la Cal Fidalgo and María Pescador Vallés board members of Asociación Autismo Huesca for their collaboration, as well as INEUP-Instituto de Neurodesarrollo y Psicología. Also, the authors would like to thank the personnel from the Psychiatry Unit of the Zamora Hospital and José Antonio del Barrio from the University of Cantabria for allowing us to conduct the assessments in their facilities.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. Funding acquisition was obtained as part of the Ph.D. project of the first author, granted to her by the University of Salamanca and Banco Santander. We declare that the funding body did not participate in the design, collection of data, analysis, interpretation, or writing of the manuscript.
Author information
Authors and Affiliations
Contributions
JAYH, DZW, and RCB: participated in conceptualization, methodology, study design, data collection, and statistical analyses. MMM: contributed to the methodology and statistical analyses. MFM and LGG: participated in the recruitment and data collection from the SSD sample. RCB and MFM: provided resources and funding acquisition. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
The present study was conducted in line with the principles of the Declaration of Helsinki. Approval was granted by the University of Salamanca Bioethics Committee (Approval Reference 386).
Consent to participate
Informed consent was obtained from the participants and from the parents of underage participants.
Consent for publication
All participants gave consent for the publication of the study results.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yon-Hernández, J.A., Wojcik, D.Z., García-García, L. et al. Neuropsychological profile of executive functions in autism spectrum disorder and schizophrenia spectrum disorders: a comparative group study in adults. Eur Arch Psychiatry Clin Neurosci 273, 719–730 (2023). https://doi.org/10.1007/s00406-022-01466-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-022-01466-w