Abstract
Psychoses in Alzheimer’s disease (AD) are associated with worse prognosis. Genetic vulnerability for schizophrenia (SCZ) may drive AD-related psychoses, yet its impact on brain constituents is still unknown. This study aimed to investigate the association between polygenic risk scores (PRSs) for SCZ and psychotic experiences (PE) and grey matter (GM) volume in patients with AD with (AD-PS) and without (AD-NP) psychosis. Clinical, genetic and T1-weighted MRI data for 800 participants were extracted from the ADNI database: 203 healthy controls, 121 AD-PS and 476 AD-NP. PRSs were calculated using a Bayesian approach and analysed at ten p-value thresholds. Standard voxel-based morphometry was used to process MRI data. Logistic regression models including both PRSs for SCZ and PE, and an AD-PRS were used to predict psychosis in AD. Associations between PRSs and GM volume were investigated in the whole sample and the three groups independently. Only the AD-PRS predicted psychosis in AD. Inconsistent associations between the SCZ-PRS and PE-PRS and GM volumes were found across groups. The SCZ-PRS was negatively associated with medio-temporal/subcortical volumes and positively with medial/orbitofrontal volumes in the AD-PS group. Only medio-temporal areas were more atrophic in the AD-PS group, while there was no significant correlation between psychosis severity and GM volume. Although not associated with psychoses, the SCZ-PRS was correlated with smaller medio-temporal and larger orbitofrontal volumes in AD-PS. Similar alterations have also been observed in SCZ patients. This finding suggest a possible disconnection between these regions associated with psychoses in more advanced AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is characterised by a heterogeneous symptomatic presentation, commonly including behavioural disturbance along with cognitive decline. About 30% of patients with AD present with psychotic symptoms, i.e. either delusions or hallucinations [1]. Both delusions and hallucinations have been found to emerge in advanced disease stages [2], although there may be differences in the temporal onset of specific psychotic symptoms [3]. Psychoses have been associated with poorer health outcomes in people with AD, such as worse cognitive decline [4], higher risk of hospitalisation and greater distress in patients and carers [5].
These detrimental effects may be partially explained by psychotic symptoms reflecting pathology-related cerebral changes in AD, including: increased concentration of hyperphosphorylated tau protein in different frontal areas [6, 7]; macrostructural and functional brain alterations across a range of areas including bilateral frontal, parietal and striatal regions [8]. Serra et al. [9] found that severity of delusions was negatively associated with the volume of the right hippocampus and middle frontal gyrus. Moreover, there is evidence that patients with AD and delusions have stronger functional connectivity in frontal regions [10] and weaker functional connectivity in the left inferior parietal lobule [11]. Hallucinations in AD have also been associated with cortical thinning in the supramarginal gyrus [12] and with smaller volume and hypometabolism in right insular, superior temporal and prefrontal areas [13]. Such variability may be due to the fact that different psychotic symptoms may be driven by partially distinct neuropathological processes and potentially by pathological changes unrelated to AD, such as Lewy bodies, vascular damage and leukoencephalopathy, that might explain psychotic symptoms in this clinical population [14].
Carriers of the Apolipoprotein E (APOE) ε4 allele, i.e. the strongest genetic risk factor for sporadic late-onset AD [15,16,17], appear at higher risk of AD-related psychosis, although this finding has not been replicated by all studies [18]. Several other genes, involved in a variety of functions but mostly linked to schizophrenia (SCZ), also appear linked to psychosis status in AD, although evidence is varied [19,20,21,22]. In fact, genome-wide association studies (GWAS) have highlighted significant associations between psychosis in AD and multiple SNPs, including the APOE gene [23], supporting the hypothesis that a multifaceted genetic underpinning may underlie this phenotype.
These findings altogether suggest the existence of a genetic association between SCZ and AD-related psychosis, probably driven by pleiotropic effects of multiple genes [24]. To investigate such claims, recent studies used polygenic risk scores (PRSs) for SCZ to predict psychosis in patients with AD. DeMichele-Sweet et al. [25] found that these symptoms were positively associated with a subset of trait-specific SNPs, but negatively associated with SCZ-PRS. A recent meta-analysis, however, observed that higher SCZ-PRS predicted psychosis in AD, although associations in individual cohorts were not consistent [26]. Further support for potential pleiotropic effects of SCZ-related genes comes from a recent study that found a SCZ-PRS to be associated with psychosis in Huntington’s disease [27].
Different lines of research converge to suggest that the potential biological correlates of psychotic symptoms in people with AD are complex and possibly determined by multiple factors. However, to date no studies have investigated whether genetic risks (e.g. SCZ-PRS) are associated not only with behavioural traits (e.g. psychosis) but also with brain parameters in people with AD. For this reason, the aim of this exploratory study, which is the first of its kind to the best of our knowledge, was to use data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) to: 1) test weather a PRS for SCZ and a novel PRS for psychotic experiences in the general population would be associated with risk of psychosis in patients with AD (higher PRSs were expected to be associated with higher risk); 2) investigate whether the two PRSs were differentially associated with regional grey matter (GM) volume in two groups of patients with AD with and without psychosis.
Methods and materials
Participants
A sample of 812 individuals with genotyping data were initially screened for inclusion. Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu).Footnote 1 Institutional review boards of each site involved in ADNI approved the study protocol and all participants provided written informed consent. Approval for secondary analyses of this dataset was granted by the Research Committee of Brunel University London (reference number 30422-TISS-Jul/2021- 33,453–2). Participants were included on the basis of the availability of genetic, MRI, neuropsychiatric and cognitive assessments. The lack of any of the abovementioned assessments and a history of previous chronic psychiatric diagnosis represented exclusion criteria for this study.
In the initial sample 601 participants received a diagnosis of either MCI or dementia due to AD, while 211 participants were healthy controls (HC), since no evidence of cognitive decline was detected longitudinally at any of the available follow-up time points. Two groups of patients were identified: 121 presenting with psychosis (AD-PS), either delusions or hallucinations recorded by means of either the Neuropsychiatric Inventory (NPI) [28] or the NPI-Questionnaire [29], and 480 with no evidence of psychosis at any time-point (AD-NP). Six HC were also found to present with psychosis and, therefore, were discarded due to concerns about the presence of potential late-onset psychosis unrelated to AD pathology. Subsequently, 5 couples of siblings were identified, hence one participant per couple was removed from our analysis to avoid biases due to relatedness [30]. Finally, one patient without psychosis was removed because no MRI data were available. The final sample consisted of a total of 800 participants: 203 HC, 476 AD-NP (383 with MCI and 83 with dementia) and 121 AD-PS (49 with MCI and 72 with dementia). To maximise our sample size and potential transferability of results [31], all participants were retained, including 6.5% from a minority ethnic background (52 non-white out of 800), although there was no significant difference in ethnicity distribution across groups (χ2 = 9.60, p = 0.65).
Clinical and cognitive data
Severity of neuropsychiatric symptoms was assessed by means of the NPI/NPI-Q, according to availability. Differently from the NPI-Q, the NPI assessment provides a total score that combines information about severity and frequency of symptoms. To quantify neuropsychiatric symptoms homogenously across participants, all NPI scores were converted into NPI-Q-like scores.
To characterise the cognitive profile of the sample, scores on a series of cognitive tests collected at the time-point closest to the neuropsychiatric assessment were also extracted. The following tests were included: Mini Mental State Examination (MMSE) [32], Clock Drawing Test, both free drawing and copy [33], Logical Memory Test, both immediate and delayed recall [34], Category Fluency Test—animals [35] and completion time of part A of the Trail Making Test [36].
Genetic data and polygenic risk scores
Apolipoprotein E (APOE) genotype status for all participants was available in the ADNI database. Genotyping was carried out by ADNI using an Illumina OmniExpress array [37]. Genotype data were curated to extract common high-quality autosomal markers using PLINKv2.0 [38]. Quality control parameters were 90% call rate, 5% minor allele frequency and Hardy–Weinberg equilibrium mid-p value 10–6. A total of 1.3 million single nucleotide polymorphisms (SNPs) passed quality control. From the quality-controlled genotype data, genetic principal components (PCs) were generated using PC-AiR [39] and the first 10 were used as covariates for regression analyses. Robust relatedness estimates, corrected for 3 PCs, were generated using PC-Relate [40]. Both approaches are implemented in the “GENESIS” R package [41].
Three polygenic risk scores (PRS) were calculated for each participant using different training sets and GWAS summary statistics: a schizophrenia PRS (SCZ-PRS) [42], one for psychotic experiences in the general population (PE-PRS) [43] and one for AD (AD-PRS) [44]. Only SNPs with imputation information content (INFO) scores greater than 0.9 were used and duplicate SNPs were removed. To our knowledge, participants in ADNI were not included in any of the discovery GWAS used to calculate PRSs.
The PE-PRS was investigated as an index complementary to the SCZ-PRS since the limited literature in this field has highlighted contrasting results [25, 26] and the development of psychoses in psychiatric disorders (and, sporadically, among the general non-psychiatric population) appears to have a different genetic basis [43]. The AD-PRS, instead, was included since AD-related risk factors have been found to contribute also to psychosis in this population [15,16,17].
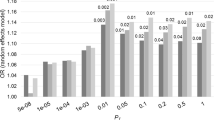
PRSs were generated using a Bayesian approach using continuous shrinkage priors (PRS-CS) [45]. After merging the data with a linkage disequilibrium reference based on the 1000 Genomes EUR samples, 334,976 SNPs were retained for SCZ, 446,852 SNPs for PE and 455,027 for AD. From each of those SNPs a Bayesian posterior effect size was calculated. The shrinkage parameter was set to φ = 1 for SCZ [46] and inferred using PRS-CS-auto for PE and AD. Finally, posterior effect sizes were used to calculate PRSs in PRSice v2 [47] without pruning at 10 GWAS p value thresholds (PT): 5 × 10–8, 1 × 10–6, 1 × 10–5, 0.001, 0.001, 0.01, 0.05, 0.1, 0.5 and 1. However, no SNPs were retained during the calculation of the PE-PRS at the two most conservative PT (5 × 10–8 and 1 × 10–6) due to the more modest power of this GWAS. PRSs were standardised (centring by mean and dividing by one standard deviation) to be used in the analysis.
PRS scores were then used to predict psychosis status in patients (AD-PS vs AD-NP) in logistic regression models including the following predictors: the psychiatric PRS (either SCZ-PRS or PE-PRS), the AD-PRS and the interaction factor between psychiatric PRS and AD-PRS, at the 10 different PT listed above. Moreover, the first 10 genetic PCs were used as covariates in the regression models to control for any potential effects of population stratification. Since psychoses mainly occur at an advanced disease stage, the same models were re-run including also the MMSE as an additional covariate to control for disease severity. The significance threshold was set at p < 0.05. Goodness of fit of the logistic regression models was determined by means of C statistics, a measure equivalent to the Area Under the Receiver Operating Characteristic curve [AUROC; 48].
A secondary logistic regression analysis was run to test the association between the AD-PRS and AD diagnosis.
MRI data and pre-processing
The structural T1-weighted MRI scan collected at the time-point closest to the neuropsychiatric assessment was selected for each participant. All MRI data were acquired as specified in the ADNI MRI protocol [49] at either 1.5 T (n = 248) or 3 T (n = 552). Pooling of MRI data acquired at different MR field strengths has been previously shown to be a valid approach with small effects on neurovolumetric quantifications [50,51,52]. The steps of the standard voxel-based morphometry (VBM) protocol [53] were carried out with Matlab (Mathworks Inc., UK) and Statistical Parametric Mapping (SPM) 12 (Wellcome Centre for Human Neuroimaging, London, UK): 1) images were reoriented to the bi-commissural axis; 2) reoriented images were segmented to separate 3 tissues, i.e. GM, white matter and cerebro-spinal fluid; 3) GM maps were modulated and then registered to the standard International Consortium of Brain Mapping (ICBM) template in the MNI space; and finally, 4) normalised images were smoothed with an 8 mm full-width at half-maximum Gaussian kernel. The global volume of each tissue map was quantified using SPM12 and, finally, the total intracranial volume (TIV) was calculated for each participant by summing the values of all 3 extracted tissues.
An ANOVA was used to compare GM volumes across groups and three t-tests were implemented for pair-wise post hoc comparisons (HC vs AD-NP, HC vs AD-PS and AD-PS vs AD-NP). Multiple regression models were created to test the association between SCZ-PRS and PE-PRS (at all PT) and GM volume in the whole sample (n = 800) and in the three groups independently (HC, AD-PS and AD-NP). The first 10 genetic PCs, age, education, sex, magnetic field strength, TIV and GM ratio (i.e. GM volume/TIV) and testing site were included as covariates in all VBM models. The same regression analyses were run to assess also the association between AD-PRS and regional GM volume. The cluster-forming significance threshold was set at p < 0.001 and results were corrected for multiple comparisons at cluster level (p < 0.05 FWE-corrected).
Subsequently, post hoc exploratory analyses were carried out on twenty-six GM regions of interest (ROIs), 13 ROIs in each hemisphere, selected on the basis of the VBM results reflecting the association between the SCZ-PRS and GM volume in the AD-PS group, to investigate 1) volumetric differences between patient groups with MANCOVA models and 2) associations between ROI volumes and psychosis severity in the AD-PS group with multiple regression. The Automated Anatomical Labelling (AAL) atlas 2 [54] was used to extract ROI volumes of: hippocampus, amygdala, parahippocampal gyrus, rectus gyrus, medial prefrontal cortex, middle and superior frontal gyri (orbital parts), middle and superior temporal gyri, fusiform gyrus, globus pallidus, middle and inferior occipital gyri. The same covariates included in the VBM models were used and Bonferroni correction for multiple comparisons was applied to the significance threshold (p < 0.0019).
Statistical analyses
Demographic and clinical characteristics were compared across groups using ANOVA for continuous variables, with Bonferroni correction for post hoc tests, and χ2 for categorical variables.
All analyses were carried out using R (www.r-project.org/) and robust standard errors were estimated to calculate 95% confidence intervals of odds ratios (ORs) resulting from logistic regressions [55] (see also: sandwich.r-forge.r-project.org/). VBM analyses were carried out using Statistical Parametric Mapping 12 (Wellcome Centre for Human Neuroimaging, London, UK).
Results
Clinical profile
The AD-PS group was significantly older than both HC and AD-NP groups and had fewer years of education than the HC group, although differences were only marginally significant (Table 1). Differences in sex distributions were also found across groups: the AD-PS group included more men than the other groups. Significant associations were found between APOE ε4 genotype and both diagnosis and psychosis, since the proportion of ε4 carriers was higher in the AD-PS than in the AD-NP group (χ2 = 16.04, p < 0.001).
Overall, both patient groups showed more severe neuropsychiatric symptoms and worse cognitive performance than the HC group. However, the AD-PS group had a more severe clinical profile than the AD-NP group characterised by higher NPI-Q scores (Table 2), worse cognitive deficits and lower GM ratio (i.e. less GM tissue available in proportion to head size). In fact, only 17.4% of patients in the AD-NP group had dementia, compared to 59.5% of the patients in the AD-PS group.
Association between PRSs and psychosis status
All logistic regression models significantly predicted psychosis in AD, but only the AD-PRS was significantly associated with psychosis across all PT (Table 3). When the analyses were run including the MMSE, this variable was the only one significantly associated with psychotic status across patients. The value of the C statistics was about 0.65 for all models that included only PRSs, while it increased to about 0.81 when the MMSE was entered as a covariate.
Logistic regression analysis on diagnosis prediction showed that the AD-PRS was significantly associated with higher risk of AD at all PT (Supplementary Information – Table S1).
Association between PRSs and GM volume
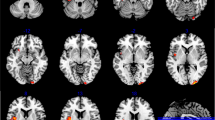
VBM ANOVA analysis showed significant differences across groups in bilateral medio-temporal areas. As expected, post hoc independent-sample t-tests found that both patients groups showed bilateral GM atrophy in medio-temporal areas when compared with the HC group (Fig. 1). Moreover, atrophy was significantly more severe in the AD-PS than in the AD-NP group (peak coordinates in Supplementary Information—Table S2).
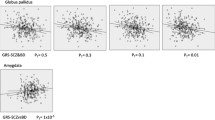
VBM regression analyses found that higher SCZ-PRS values were associated mainly, but inconsistently across PT, with smaller volume in occipital areas in the whole sample and in the HC group (Fig. 2) (Supplementary Information—Table S3 and Figure S1). In the AD-PS groups, instead, the SCZ-PRS was positively associated with the volume of bilateral medial prefrontal (mPFC) and orbitofrontal cortices (OFC) and negatively with the volume of right-lateralised medio-temporal and basal ganglia GM areas, as well as some occipital clusters, but only at less conservative thresholds (PT = 0.05 and PT = 0.1) (Fig. 2 and Table 4; for a comprehensive representation of all results see Supplementary Information—Figure S2). No significant genetic-neurovolumetric associations were observed for the AD-NP group.
More inconsistent associations were observed between the PE-PRS and regional GM volumes (Supplementary Information—Table S3). In the whole sample of participants, the PE-PRS was negatively associated with the volume of left-lateralised insulo-temporal cortices and positively with the volume of a cerebellar cluster. However, the PE-PRS was positively associated with GM volume in cerebellar and occipital areas in the AD-NP group, and in cingulate and medial frontal areas in the HC group. No significant results were found for patients with psychosis.
VBM regression models investigating the effect of the AD-PRS on GM volume showed significant negative associations with clusters in bilateral medio-temporal and right inferior temporal and posterior cingulate areas, in the whole sample, and in left-lateralised medio-temporal areas, in the AD-PS group (Supplementary Information—Table S4 and Figure S3).
When volumes of GM ROIs (selected on the basis of the findings of whole brain analyses) were compared between patient groups, bilateral medial and right middle temporal areas were the only ones to be significantly more atrophic in patients with psychosis than in those without (Table 5). Severity of psychosis in the AD-PS group was not associated with any ROI volumes (Supplementary Information—Table S5).
Discussion
Genetic analyses revealed that only the AD-PRS, among all those investigated, was significantly associated with psychosis status in this sample of patients with AD, when disease severity was not accounted for. However, all the models only yielded C statistics values < 0.80, thus indicating that currently PRSs alone may have limited utility for prediction of this phenotype in a clinical setting. On the contrary, when the MMSE was included among the predictors, it emerged as the only variable significantly associated with psychosis and led to a considerable increase in model fit (C > 0.80). Associations between PRSs and neurovolumetric features, instead, were not univocal, although more consistent between the SCZ-PRS and GM volume in medio-temporal and OFC/mPFC areas in the AD-PS group.
The lack of associations between the SCZ-PRS and the clinical psychotic phenotype in our sample is in contrast with significant, although inconsistent, findings from previous investigations. Indeed, DeMichele-Sweet et al. [25] observed that psychotic symptoms in AD were associated with a set of genes but inversely with a SCZ-PRS that appeared to be protective against psychosis in their cohort. However, a recent comprehensive meta-analysis found a significant positive association between a SCZ-PRS and risk of psychosis in AD by combining 11 cohorts of patients [26]. Nevertheless, quite inconsistent results emerged in individual cohorts and across PT investigated, probably due to either limited power or differences in age, MMSE score and gender proportions across cohorts. It must be noted that the ADNI sample selected by Creese et al. [26] differs from ours as it appears smaller and the reported confidence intervals are only partially overlapping with ours. Other methodological differences may have also contributed to the divergence in results, namely: the GWAS summary statistics used to compute the SCZ-PRS, the number of genetic PCs accounted for in the analyses and the inclusion of the AD-PRS and interaction factors in our logistic regression models.
No significant results emerged for the PE-PRS, a metric that should capture non-specific risk for any type of self-reported psychotic experiences in the general population and that is associated with a range of disorders [43]. These findings may be due to the low phenotypic variance explained by the PE-PRS and, therefore, no association with AD-related psychoses are likely to be detected if these symptoms are mainly driven by a set of more disease-specific genes as proposed by DeMichele-Sweet et al. [25].
Overall, it appears that the risk of psychosis in AD may be primarily linked to genetic liability for AD, consistently with previous evidence suggesting that the APOE ε4 allele (potentially with some additional contribution of other AD risk genes) is the main fosterer of psychosis in this population [15,16,17]. In fact, the AD-PS group comprised a significantly higher proportion of ε4 carriers than the AD-NP group. After controlling for disease severity, however, this association did not survive, hence suggesting that psychotic symptoms may be more prevalent either in advanced disease stages [2] or in more severe cases with higher AD-PRS and more pronounced neurodegeneration. Consistently with this hypothesis, between-group comparisons highlighted worse cognitive decline and medio-temporal lobe atrophy for the AD-PS compared to the AD-NP group.
VBM regression analyses on the PE-PRS showed largely inconsistent positive associations with GM volume of cerebellar/occipital areas, at a conservative PT, in the whole sample and in the AD-PS group and with GM volume in the anterior cingulate in the HC group. The PE-PRS was also associated with lower GM volume in left insular/superior temporal areas in the whole sample, in line with previous findings in the general population [56] and in patients with AD [57]. However, no significant associations were detected in the AD-PS group, hence suggesting that PE-PRS may not be a contributing factor for AD-related psychoses.
The SCZ-PRS, instead, was negatively associated with the volume in occipital regions in the whole sample and in the HC group, and even in the AD-PS group at some less conservative PT. The relevance of these findings is unclear because of the inconsistency observed across the tested PRS thresholds and evidence from previous observations of a positive association between SCZ-PRS and occipital GM volume [58]. In the AD-PS group, the SCZ-PRS was associated with more reliable neural signatures, largely dissociable from those detected for the AD-PRS. While the AD-PRS was associated with lower GM volume in left-lateralised temporal areas, the SCZ-PRS was associated with larger volume in OFC/mPFC areas, involved in reward processing and social behaviours, and lower volume in right-sided medio-temporal areas, crucial for memory and emotion processing, and in the right globus pallidus, involved in motor control and executive functions. The negative associations with medio-temporal and basal ganglia volumes are in line with previous investigations on SCZ patients [59, 60], although these findings were not replicated in all studies [61, 62]. Moreover, previous observations had already shown that altered volumetric features of the OFC may be implicated in SCZ onset [63, 64].
The SCZ-PRS exerted opposite effects on medio-temporal/subcortical and frontal areas and this appears to be consistent with previous accounts [65] of GM loss and disruption in functional connectivity between medio-temporal and OFC areas associated with behavioural disturbance in a mouse model of SCZ [22]. The same pattern of alteration in hippocampus-OFC functional connectivity was also found associated with SCZ-PRS in people with SCZ and their unaffected first-degree relatives [66]. Moreover, altered functional connectivity in frontal areas including the OFC has also been observed in patients with AD and delusions [10]. Therefore, it appears that a dysfunction in the communication between medio-temporal and orbitofrontal areas, the volumes of which were associated with SCZ-PRS in the AD-PS group, may play a central role in the manifestation of psychotic symptoms also in patients with AD.
It must be noted that SCZ, differently from AD, is commonly regarded as a neurodevelopmental disorder [67] influenced by interacting genetic and environmental factors that often lead to disease onset in late adolescence/early adulthood [68, 69]. To the best of our knowledge, participants included in this study presented with psychotic symptoms only in older adulthood, after substantial AD-related pathological changes had occurred. Therefore, psychotic symptoms in AD may manifest when neurodegeneration exceeds a certain threshold in a cluster of brain areas in susceptible individuals at greater genetic risk. Psychosis may, therefore, emerge when a pattern of latent neural vulnerability in certain brain regions, shaped by polygenic risk for SCZ, is unveiled by the progression of neurodegeneration. In fact, the comparison between patient groups also showed preserved GM volume in frontal areas that was positively associated with the SCZ-PRS in the AD-PS group. Hence, patients with AD and psychosis appear to be those presenting with a neurovolumetric pattern suggestive of a disconnection between medio-temporal and OFC areas similar to that observed in SCZ [66], i.e. a combination of severe medio-temporal atrophy, mainly due to AD pathology but potentially also influenced by SCZ risk, and preserved volume in OFC/mPFC areas associated with higher polygenic risk for SCZ.
Some limitations to this work must be mentioned. First, the AD-PS group has been defined pooling all types of psychotic symptoms, but it is likely that individual symptoms may be characterised by partially distinct aetiologies, neural and neuropathological correlates [3, 70, 71]. Second, the limited sample size, especially for the HC and AD-PS groups. Third, the inclusion of 52 participants from a minority ethnic background might have introduced variability in the data not related to the phenotype of interest; however, we decided to retain these datasets in the analyses to maximise the power of this study and increase its diversity, and included 10 genetic PCs in all regression models to minimise any bias. Fourth, we only included MRI data on brain macrostructure (those available almost universally across ADNI participants) while we did not investigate functional brain MRI data; indeed altered brain activity may represent, compared with GM atrophy, a predominant neural process associated with psychosis in AD. Finally, previously it has been suggested that the SCZ-PRS (though this could in principle be extended to any PRS derived from a complex trait) is a heterogeneous construct that captures the effect of many biological pathways associated with the disorder [72]. As such, there is some evidence that restricting SCZ-PRS to sets of functionally related genes might improve its predictive capabilities in the context of neuroimaging studies [60]. However, we did not attempt these complex partitioning procedures since, to date, no large gene sets that could result in a well-powered PRS have been associated specifically with psychotic experiences.
In summary, this study provides novel findings that suggest that polygenic risk for SCZ is associated with a specific neural configuration in patients with AD and psychoses. In particular, the combination of smaller medio-temporal volumes but preserved OFC/mPFC volumes may signal a disconnection between these two systems, already implicated in SCZ, and foster the neural conditions that generate a cluster of behavioural alterations that could explain psychotic presentations in people with AD. Future investigations combining genetic as well as brain activity/metabolism data will be better placed to clarify the role of the medio-temporal-orbitofrontal disconnection in the genesis of psychoses in AD.
Data availability
All ADNI data are made publicly available.
Code availability
Not applicable.
Notes
The ADNI was launched in 2003 as a public–private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early Alzheimer’s disease (AD). For up-to-date information, see www.adni-info.org.
References
Aarsland D (2020) Epidemiology and pathophysiology of dementia-related psychosis. J Clin Psychiatry 81:AD19038BR19031C
Masters MC, Morris JC, Roe CM (2015) “Noncognitive” symptoms of early Alzheimer disease: a longitudinal analysis. Neurology 84:617–622
Fischer CE, Ismail Z, Youakim JM, Creese B, Kumar S, Nuñez N, Ryan Darby R, Di Vita A, D’Antonio F, de Lena C, McGeown WJ, Ramit R, Rasmussen J, Bell J, Wang H, Bruneau MA, Panegyres PK, Lanctôt KL, Agüera-Ortiz L, Lyketsos C, Cummings J, Jeste DV, Sano M, Devanand DP, Sweet RA, Ballard C (2020) Revisiting criteria for psychosis in Alzheimer’s disease and related dementias: toward better phenotypic classification and biomarker research. J Alzheimers Dis 73:1143–1156
Zahodne LB, Ornstein K, Cosentino S, Devanand DP, Stern Y (2015) Longitudinal relationships between Alzheimer disease progression and psychosis, depressed mood, and agitation/aggression. Am J Geriatr Psychiatry 23:130–140
Connors MH, Ames D, Woodward M, Brodaty H (2018) Psychosis and clinical outcomes in Alzheimer disease: a longitudinal study. Am J Geriatr Psychiatry 26:304–313
Murray PS, Kirkwood CM, Gray MC, Fish KN, Ikonomovic MD, Hamilton RL, Kofler JK, Klunk WE, Lopez OL, Sweet RA (2014) Hyperphosphorylated tau is elevated in Alzheimer’s disease with psychosis. J Alzheimers Dis 39:759–773
Koppel J, Acker C, Davies P, Lopez OL, Jimenez H, Azose M, Greenwald BS, Murray PS, Kirkwood CM, Kofler J, Sweet RA (2014) Psychotic Alzheimer’s disease is associated with gender-specific tau phosphorylation abnormalities. Neurobiol Aging 35:2021–2028
Mega MS, Lee L, Dinov ID, Mishkin F, Toga AW, Cummings JL (2000) Cerebral correlates of psychotic symptoms in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 69:167–171
Serra L, Perri R, Cercignani M, Spanò B, Fadda L, Marra C, Carlesimo GA, Caltagirone C, Bozzali M (2010) Are the behavioral symptoms of Alzheimer’s disease directly associated with neurodegeneration? J Alzheimers Dis 21:627–639
Tetreault AM, Phan T, Orlando D, Lyu I, Kang H, Landman B, Darby RR (2020) Network localization of clinical, cognitive, and neuropsychiatric symptoms in Alzheimer’s disease. Brain 143:1249–1260
Qian W, Fischer CE, Churchill NW, Kumar S, Rajji T, Schweizer TA (2019) Delusions in Alzheimer disease are associated with decreased default mode network functional connectivity. Am J Geriatr Psychiatry 27:1060–1068
Donovan NJ, Wadsworth LP, Lorius N, Locascio JJ, Rentz DM, Johnson KA, Sperling RA, Marshall GA (2014) Regional cortical thinning predicts worsening apathy and hallucinations across the Alzheimer disease spectrum. Am J Geriatr Psychiatry 22:1168–1179
Blanc F, Noblet V, Philippi N, Cretin B, Foucher J, Armspach JP, Rousseau F (2014) Right anterior insula: Core region of hallucinations in cognitive neurodegenerative diseases. PLoS ONE 9:e114774
Fischer CE, Qian W, Schweizer TA, Millikin CP, Ismail Z, Smith EE, Lix LM, Shelton P, Munoz DG (2016) Lewy bodies, vascular risk factors, and subcortical arteriosclerotic leukoencephalopathy, but not Alzheimer pathology, are associated with development of psychosis in Alzheimer’s disease. J Alzheimers Dis 50:283–295
de Oliveira FF, Chen ES, Smith MC, Bertolucci PH (2017) Associations of cerebrovascular metabolism genotypes with neuropsychiatric symptoms and age at onset of Alzheimer’s disease dementia. Braz J Psychiatry 39:95–103
Qian W, Fischer CE, Schweizer TA, Munoz DG (2018) Association between psychosis phenotype and APOE genotype on the clinical profiles of Alzheimer’s disease. Curr Alzheimer Res 15:187–194
Zdanys KF, Kleiman TG, MacAvoy MG, Black BT, Rightmer TE, Grey M, Garman KS, Tampi RR, Gelernter J, van Dyck CH (2007) Apolipoprotein E epsilon4 allele increases risk for psychotic symptoms in Alzheimer’s disease. Neuropsychopharmacology 32:171–179
D’Onofrio G, Panza F, Seripa D, Sancarlo D, Paris F, Cascavilla L, Urbano M, Gravina C, Fontana A, Solfrizzi V, Pellegrini F, Pilotto A (2011) The apoe polymorphism in Alzheimer’s disease patients with neuropsychiatric symptoms and syndromes. Int J Geriatr Psychiatry 26:1062–1070
Borroni B, Grassi M, Costanzi C, Zanetti M, Archetti S, Franzoni S, Caimi L, Padovani A (2007) Haplotypes in cathechol-o-methyltransferase gene confer increased risk for psychosis in Alzheimer disease. Neurobiol Aging 28:1231–1238
Carson R, Craig D, Hart D, Todd S, McGuinness B, Johnston JA, O’Neill FA, Ritchie CW, Passmore AP (2008) Genetic variation in the alpha 7 nicotinic acetylcholine receptor is associated with delusional symptoms in Alzheimer’s disease. Neuromolecular Med 10:377–384
Shah C, DeMichele-Sweet MA, Sweet RA (2017) Genetics of psychosis of Alzheimer disease. Am J Med Genet B Neuropsychiatr Genet 174:27–35
Zheng X, Demirci FY, Barmada MM, Richardson GA, Lopez OL, Sweet RA, Kamboh MI, Feingold E (2014) A rare duplication on chromosome 16p11.2 is identified in patients with psychosis in Alzheimer's disease. PLoS One 9:e111462
Hollingworth P, Sweet R, Sims R, Harold D, Russo G, Abraham R, Stretton A, Jones N, Gerrish A, Chapman J, Ivanov D, Moskvina V, Lovestone S, Priotsi P, Lupton M, Brayne C, Gill M, Lawlor B, Lynch A, Craig D, McGuinness B, Johnston J, Holmes C, Livingston G, Bass NJ, Gurling H, McQuillin A, Consortium11 G, Group12 tNIoAL-OAsDFS, Holmans P, Jones L, Devlin B, Klei L, Barmada MM, Demirci FY, DeKosky ST, Lopez OL, Passmore P, Owen MJ, O'Donovan MC, Mayeux R, Kamboh MI, Williams J (2012) Genome-wide association study of Alzheimer's disease with psychotic symptoms. Mol Psychiatry 17:1316–1327
Bellou E, Stevenson-Hoare J, Escott-Price V (2020) Polygenic risk and pleiotropy in neurodegenerative diseases. Neurobiol Dis 142:104953
DeMichele-Sweet MAA, Weamer EA, Klei L, Vrana DT, Hollingshead DJ, Seltman HJ, Sims R, Foroud T, Hernandez I, Moreno-Grau S, Tárraga L, Boada M, Ruiz A, Williams J, Mayeux R, Lopez OL, Sibille EL, Kamboh MI, Devlin B, Sweet RA (2018) Genetic risk for schizophrenia and psychosis in Alzheimer disease. Mol Psychiatry 23:963–972
Creese B, Vassos E, Bergh S, Athanasiu L, Johar I, Rongve A, Medbøen IT, Vasconcelos Da Silva M, Aakhus E, Andersen F, Bettella F, Braekhus A, Djurovic S, Paroni G, Proitsi P, Saltvedt I, Seripa D, Stordal E, Fladby T, Aarsland D, Andreassen OA, Ballard C, Selbaek G, Initiative obotAcatAsDN (2019) Examining the association between genetic liability for schizophrenia and psychotic symptoms in Alzheimer's disease. Transl Psychiatry 9:273
Ellis N, Tee A, McAllister B, Massey T, McLauchlan D, Stone T, Correia K, Loupe J, Kim KH, Barker D, Hong EP, Chao MJ, Long JD, Lucente D, Vonsattel JPG, Pinto RM, Elneel KA, Ramos EM, Mysore JS, Gillis T, Wheeler VC, Medway C, Hall L, Kwak S, Sampaio C, Ciosi M, Maxwell A, Chatzi A, Monckton DG, Orth M, Landwehrmeyer GB, Paulsen JS, Shoulson I, Myers RH, van Duijn E, Rickards H, MacDonald ME, Lee JM, Gusella JF, Jones L, Holmans P (2020) Genetic risk underlying psychiatric and cognitive symptoms in Huntington’s disease. Biol Psychiatry 87:857–865
Cummings JL (1997) The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology 48:S10-16
Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, Lopez OL, DeKosky ST (2000) Validation of the NPI-Q, a brief clinical form of the neuropsychiatric inventory. J Neuropsychiatry Clin Neurosci 12:233–239
Choi SW, Mak TS, O’Reilly PF (2020) Tutorial: a guide to performing polygenic risk score analyses. Nat Protoc 15:2759–2772
Mills MC, Rahal C (2020) The GWAS diversity monitor tracks diversity by disease in real time. Nat Genet 52:242–243
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Goodglass H, Kaplan E (1983) The assessment of aphasia and related disorders. Lea & Febiger, Philadelphia
Wechsler D (1987) WMS-R Wechsler Memory Scale - Revised manual. The Psychological Corporation, Harcourt Brace Jovanovich Inc, New York
Butters N, Granholm E, Salmon DP, Grant I, Wolfe J (1987) Episodic and semantic memory: a comparison of amnesic and demented patients. J Clin Exp Neuropsychol 9:479–497
Reitan RM (1958) Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills 8:271–276
Saykin AJ, Shen L, Yao X, Kim S, Nho K, Risacher SL, Ramanan VK, Foroud TM, Faber KM, Sarwar N, Munsie LM, Hu X, Soares HD, Potkin SG, Thompson PM, Kauwe JS, Kaddurah-Daouk R, Green RC, Toga AW, Weiner MW, Initiative ADN (2015) Genetic studies of quantitative MCI and AD phenotypes in ADNI: progress, opportunities, and plans. Alzheimers Dement 11:792–814
Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ (2015) Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 4:7
Conomos MP, Miller MB, Thornton TA (2015) Robust inference of population structure for ancestry prediction and correction of stratification in the presence of relatedness. Genet Epidemiol 39:276–293
Conomos MP, Reiner AP, Weir BS, Thornton TA (2016) Model-free estimation of recent genetic relatedness. Am J Hum Genet 98:127–148
Gogarten SM, Sofer T, Chen H, Yu C, Brody JA, Thornton TA, Rice KM, Conomos MP (2019) Genetic association testing using the genesis r/bioconductor package. Bioinformatics 35:5346–5348
Pardiñas AF, Holmans P, Pocklington AJ, Escott-Price V, Ripke S, Carrera N, Legge SE, Bishop S, Cameron D, Hamshere ML, Han J, Hubbard L, Lynham A, Mantripragada K, Rees E, MacCabe JH, McCarroll SA, Baune BT, Breen G, Byrne EM, Dannlowski U, Eley TC, Hayward C, Martin NG, McIntosh AM, Plomin R, Porteous DJ, Wray NR, Caballero A, Geschwind DH, Huckins LM, Ruderfer DM, Santiago E, Sklar P, Stahl EA, Won H, Agerbo E, Als TD, Andreassen OA, Bækvad-Hansen M, Mortensen PB, Pedersen CB, Børglum AD, Bybjerg-Grauholm J, Djurovic S, Durmishi N, Pedersen MG, Golimbet V, Grove J, Hougaard DM, Mattheisen M, Molden E, Mors O, Nordentoft M, Pejovic-Milovancevic M, Sigurdsson E, Silagadze T, Hansen CS, Stefansson K, Stefansson H, Steinberg S, Tosato S, Werge T, Consortium G, Consortium C, Collier DA, Rujescu D, Kirov G, Owen MJ, O'Donovan MC, Walters JTR (2018) Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection. Nat Genet 50:381-389
Legge SE, Jones HJ, Kendall KM, Pardiñas AF, Menzies G, Bracher-Smith M, Escott-Price V, Rees E, Davis KAS, Hotopf M, Savage JE, Posthuma D, Holmans P, Kirov G, Owen MJ, O’Donovan MC, Zammit S, Walters JTR (2019) Association of genetic liability to psychotic experiences with neuropsychotic disorders and traits. JAMA Psychiat 76:1256–1265
Jansen IE, Savage JE, Watanabe K, Bryois J, Williams DM, Steinberg S, Sealock J, Karlsson IK, Hägg S, Athanasiu L, Voyle N, Proitsi P, Witoelar A, Stringer S, Aarsland D, Almdahl IS, Andersen F, Bergh S, Bettella F, Bjornsson S, Brækhus A, Bråthen G, de Leeuw C, Desikan RS, Djurovic S, Dumitrescu L, Fladby T, Hohman TJ, Jonsson PV, Kiddle SJ, Rongve A, Saltvedt I, Sando SB, Selbæk G, Shoai M, Skene NG, Snaedal J, Stordal E, Ulstein ID, Wang Y, White LR, Hardy J, Hjerling-Leffler J, Sullivan PF, van der Flier WM, Dobson R, Davis LK, Stefansson H, Stefansson K, Pedersen NL, Ripke S, Andreassen OA, Posthuma D (2019) Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat Genet 51:404–413
Ge T, Chen CY, Ni Y, Feng YA, Smoller JW (2019) Polygenic prediction via bayesian regression and continuous shrinkage priors. Nat Commun 10:1776
Zheutlin AB, Dennis J, Karlsson Linnér R, Moscati A, Restrepo N, Straub P, Ruderfer D, Castro VM, Chen CY, Ge T, Huckins LM, Charney A, Kirchner HL, Stahl EA, Chabris CF, Davis LK, Smoller JW (2019) Penetrance and pleiotropy of polygenic risk scores for schizophrenia in 106,160 patients across four health care systems. Am J Psychiatry 176:846–855
Choi SW, O'Reilly PF (2019) Prsice-2: Polygenic risk score software for biobank-scale data. Gigascience 8:giz082
Hosmer DW, Lemeshow S (2000) Applied logistic regression, 2nd edn. John Wiley & Sons, New York, NY
Jack Jr CR, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, Borowski B, Britson PJ, L Whitwell J, Ward C, Dale AM, Felmlee JP, Gunter JL, Hill DL, Killiany R, Schuff N, Fox-Bosetti S, Lin C, Studholme C, DeCarli CS, Krueger G, Ward HA, Metzger GJ, Scott KT, Mallozzi R, Blezek D, Levy J, Debbins JP, Fleisher AS, Albert M, Green R, Bartzokis G, Glover G, Mugler J, Weiner MW (2008) The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging 27:685–691
Marchewka A, Kherif F, Krueger G, Grabowska A, Frackowiak R, Draganski B, AsDN I (2014) Influence of magnetic field strength and image registration strategy on voxel-based morphometry in a study of Alzheimer’s disease. Hum Brain Mapp 35:1865–1874
Schmitter D, Roche A, Maréchal B, Ribes D, Abdulkadir A, Bach-Cuadra M, Daducci A, Granziera C, Klöppel S, Maeder P, Meuli R, Krueger G, AsDN I (2014) An evaluation of volume-based morphometry for prediction of mild cognitive impairment and Alzheimer’s disease. Neuroimage Clin 7:7–17
Stonnington CM, Tan G, Klöppel S, Chu C, Draganski B, Jack CR Jr, Chen K, Ashburner J, Frackowiak RS (2008) Interpreting scan data acquired from multiple scanners: a study with Alzheimer’s disease. Neuroimage 39:1180–1185
Ashburner J, Friston KJ (2000) Voxel-based morphometry–the methods. Neuroimage 11:805–821
Rolls ET, Joliot M, Tzourio-Mazoyer N (2015) Implementation of a new parcellation of the orbitofrontal cortex in the automated anatomical labeling atlas. Neuroimage 122:1–5
Zeileis A, Köll S, Graham N (2020) Various versatile variances: An object-oriented implementation of clustered covariances in r. J Stat Softw 95:1–36
Alloza C, Blesa-Cábez M, Bastin ME, Madole JW, Buchanan CR, Janssen J, Gibson J, Deary IJ, Tucker-Drob EM, Whalley HC, Arango C, McIntosh AM, Cox SR, Lawrie SM (2020) Psychotic-like experiences, polygenic risk scores for schizophrenia, and structural properties of the salience, default mode, and central-executive networks in healthy participants from UK Biobank. Transl Psychiatry 10:122
Tascone LDS, Payne ME, MacFall J, Azevedo D, de Castro CC, Steffens DC, Busatto GF, Bottino CMC (2017) Cortical brain volume abnormalities associated with few or multiple neuropsychiatric symptoms in Alzheimer’s disease. PLoS ONE 12:e0177169
Ranlund S, Rosa MJ, de Jong S, Cole JH, Kyriakopoulos M, Fu CHY, Mehta MA, Dima D (2018) Associations between polygenic risk scores for four psychiatric illnesses and brain structure using multivariate pattern recognition. Neuroimage Clin 20:1026–1036
Alnæs D, Kaufmann T, van der Meer D, Córdova-Palomera A, Rokicki J, Moberget T, Bettella F, Agartz I, Barch DM, Bertolino A, Brandt CL, Cervenka S, Djurovic S, Doan NT, Eisenacher S, Fatouros-Bergman H, Flyckt L, Di Giorgio A, Haatveit B, Jönsson EG, Kirsch P, Lund MJ, Meyer-Lindenberg A, Pergola G, Schwarz E, Smeland OB, Quarto T, Zink M, Andreassen OA, Westlye LT, Consortium KSP (2019) Brain heterogeneity in schizophrenia and its association with polygenic risk. JAMA Psychiatry 76:739-748
Grama S, Willcocks I, Hubert JJ, Pardiñas AF, Legge SE, Bracher-Smith M, Menzies GE, Hall LS, Pocklington AJ, Anney RJL, Bray NJ, Escott-Price V, Caseras X (2020) Polygenic risk for schizophrenia and subcortical brain anatomy in the UK Biobank cohort. Transl Psychiatry 10:309
Reus LM, Shen X, Gibson J, Wigmore E, Ligthart L, Adams MJ, Davies G, Cox SR, Hagenaars SP, Bastin ME, Deary IJ, Whalley HC, McIntosh AM (2017) Association of polygenic risk for major psychiatric illness with subcortical volumes and white matter integrity in UK Biobank. Sci Rep 7:42140
Van der Auwera S, Wittfeld K, Shumskaya E, Bralten J, Zwiers MP, Onnink AM, Usberti N, Hertel J, Völzke H, Völker U, Hosten N, Franke B, Grabe HJ (2017) Predicting brain structure in population-based samples with biologically informed genetic scores for schizophrenia. Am J Med Genet B Neuropsychiatr Genet 174:324–332
Harris JM, Moorhead TW, Miller P, McIntosh AM, Bonnici HM, Owens DG, Johnstone EC, Lawrie SM (2007) Increased prefrontal gyrification in a large high-risk cohort characterizes those who develop schizophrenia and reflects abnormal prefrontal development. Biol Psychiatry 62:722–729
Lacerda AL, Hardan AY, Yorbik O, Vemulapalli M, Prasad KM, Keshavan MS (2007) Morphology of the orbitofrontal cortex in first-episode schizophrenia: Relationship with negative symptomatology. Prog Neuropsychopharmacol Biol Psychiatry 31:510–516
Bristow GC, Thomson DM, Openshaw RL, Mitchell EJ, Pratt JA, Dawson N, Morris BJ (2020) 16p11 duplication disrupts hippocampal-orbitofrontal-amygdala connectivity, revealing a neural circuit endophenotype for schizophrenia. Cell Rep 31:107536
Liu S, Li A, Liu Y, Yan H, Wang M, Sun Y, Fan L, Song M, Xu K, Chen J, Chen Y, Wang H, Guo H, Wan P, Lv L, Yang Y, Li P, Lu L, Yan J, Wang H, Zhang H, Wu H, Ning Y, Zhang D, Jiang T, Liu B (2020) Polygenic effects of schizophrenia on hippocampal grey matter volume and hippocampus-medial prefrontal cortex functional connectivity. Br J Psychiatry 216:267–274
Fatemi SH, Folsom TD (2009) The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr Bull 35:528–548
Buoli M, Serati M, Caldiroli A, Cremaschi L, Altamura AC (2017) Neurodevelopmental versus neurodegenerative model of schizophrenia and bipolar disorder: comparison with physiological brain development and aging. Psychiatr Danub 29:24–27
Bolhuis K, Tiemeier H, Jansen PR, Muetzel RL, Neumann A, Hillegers MHJ, van den Akker ETL, van Rossum EFC, Jaddoe VWV, Vernooij MW, White T, Kushner SA (2019) Interaction of schizophrenia polygenic risk and cortisol level on pre-adolescent brain structure. Psychoneuroendocrinology 101:295–303
Ismail Z, Nguyen MQ, Fischer CE, Schweizer TA, Mulsant BH (2012) Neuroimaging of delusions in Alzheimer’s disease. Psychiatry Res 202:89–95
Naasan G, Shdo SM, Rodriguez EM, Spina S, Grinberg L, Lopez L, Karydas A, Seeley WW, Miller BL, Rankin KP (2021) Psychosis in neurodegenerative disease: Differential patterns of hallucination and delusion symptoms. Brain 144:999–1012
Rammos A, Gonzalez LAN, The Schizophrenia Working Group of the Psychiatric Genomics Consortium 2, Weinberger DR, Mitchell KJ, Nicodemus KK (2019) The role of polygenic risk score gene-set analysis in the context of the omnigenic model of schizophrenia. Neuropsychopharmacology 44:1562-1569
Acknowledgements
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. *Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
Funding
Not applicable.
Author information
Authors and Affiliations
Consortia
Contributions
RM conceived and designed the study, selected and analysed data, contributed to interpretation of the results, prepared and approved the final version of the manuscript; AFP contributed to designing the study and analyses of data, contributed to interpretation of the results, revised and approved the final version of the manuscript; AV conceived and designed the study, contributed to interpretation of the results, revised and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Not applicable.
Ethics approval
Institutional review boards of each site involved in ADNI approved the study protocol. Approval for secondary analyses of this dataset was granted by the Research Committee of Brunel University of London (reference number 30422-TISS-Jul/2021- 33453–2).
Consent to participate
All participants provided written informed consent to take part in ADNI.
Consent for publication
All ADNI participants provided written informed consent to sharing their data with the scientific community, that includes also publications.
Additional information
List of authors present in Alzheimer’s Disease Neuroimaging Initiative are listed in acknowledgement.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Manca, R., Pardiñas, A.F., Venneri, A. et al. The neural signatures of psychoses in Alzheimer’s disease: a neuroimaging genetics approach. Eur Arch Psychiatry Clin Neurosci 273, 253–267 (2023). https://doi.org/10.1007/s00406-022-01432-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-022-01432-6