Abstract
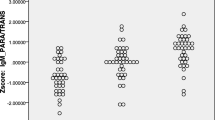
The aim of this research was to assess whether or not changes in the concentrations of serum zonulin and claudin-5 in patients with schizophrenia could have etiopathogenetic importance. In previous studies, the data regarding the relationship between intestinal and blood–brain barrier (BBB) permeability and the etiology of schizophrenia have been limited. In this study, we assumed that there may be a difference in serum zonulin and claudin-5 levels in patients with schizophrenia, which may affect the severity of the disease. Fifty schizophrenia patients and 50 healthy controls were included in this study. The patients were administered the Positive Symptoms Assessment Scale (SAPS) and Negative Symptoms Assessment Scale (SANS) to determine the severity of symptoms. Venous blood samples were collected, and the serum zonulin and claudin-5 levels were measured. The mean serum zonulin levels were significantly increased in patients with schizophrenia when compared to the control group. Serum claudin-5 levels were decreased in the schizophrenia patients when compared to the controls. The present study indicates that zonulin is increased and claudin-5 is decreased in patients with schizophrenia. These findings extend the existing knowledge on the dysregulation of intestinal permeability, especially zonulin, and BBB, especially claudin-5, and show that both proteins may be involved in the etiopathogenesis of schizophrenia.

Similar content being viewed by others
References
Andreasen NC (1990) Methods for assessing positive and negative symptoms. Mod Probl Pharmacopsychiatry 24:73–88. https://doi.org/10.1159/000418013
Arinami T (2006) Analyses of the associations between the genes of 22q11 deletion syndrome and schizophrenia. J Hum Genet 51:1037–1045. https://doi.org/10.1007/s10038-006-0058-5
Asmar RE, Panigrahi P, Bamford P et al (2002) Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology 123:1607–1615. https://doi.org/10.1053/gast.2002.36578
Barber GS, Sturgeon C, Fasano A et al (2019) Elevated zonulin, a measure of tight-junction permeability, may be implicated in schizophrenia. Schizophr Res 211:111–112. https://doi.org/10.1016/j.schres.2019.07.006
Bassett AS, Chow EWC (2008) Schizophrenia and 22q11.2 deletion syndrome. Curr Psychiatry Rep 10:148–157
Drago S, El Asmar R, Di Pierro M et al (2006) Gliadin, zonulin and gut permeability: effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand J Gastroenterol 41:408–419. https://doi.org/10.1080/00365520500235334
Erkoç Ş, Arkonac O, Ataklı C, Özmen E (1991) Pozitif Semptomları Değerlendirme Ölçeğinin Güvenilirliği ve Geçerliliği. Dusunen Adam 4:20–24
Erkoç Ş, Arkonac O, Ataklı C, Özmen E (1991) Negatif Semptomları Değerlendirme Ölçeğinin Güvenilirliği ve Geçerliliği. Dusunen Adam 4:14–15
Esnafoglu E, Cırrık S, Ayyıldız SN et al (2017) Increased serum zonulin levels as an intestinal permeability marker in autistic subjects. J Pediatr 188:240–244. https://doi.org/10.1016/j.jpeds.2017.04.004
Fasano A (2011) Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev 91:151–175. https://doi.org/10.1152/physrev.00003.2008
Fasano A (2012) Zonulin, regulation of tight junctions, and autoimmune diseases. Ann NY Acad Sci 1258:25–33. https://doi.org/10.1111/j.1749-6632.2012.06538.x
Fasano A, Hill I (2017) Serum Zonulin, Gut Permeability, and the pathogenesis of autism spectrum disorders: cause, effect, or an epiphenomenon? J Pediatr 188:15–17. https://doi.org/10.1016/j.jpeds.2017.05.038
Fasano A, Uzzau S, Fiore C, Margaretten K (1997) The enterotoxic effect of zonula occludens toxin on rabbit small intestine involves the paracellular pathway. Gastroenterology 112:839–846. https://doi.org/10.1053/gast.1997.v112.pm9041245
Greene C, Hanley N, Campbell M (2019) Claudin-5: gatekeeper of neurological function. Fluids Barriers CNS 16:3. https://doi.org/10.1186/s12987-019-0123-z
Greene C, Kealy J, Humphries MM et al (2018) Dose-dependent expression of claudin-5 is a modifying factor in schizophrenia. Mol Psychiatry 23:2156–2166. https://doi.org/10.1038/mp.2017.156
Harrison PJ, Owen MJ (2003) Genes for schizophrenia? Recent findings and their pathophysiological implications. Lancet 361:417–419. https://doi.org/10.1016/S0140-6736(03)12379-3
Ishiguro H, Imai K, Koga M et al (2008) Replication study for associations between polymorphisms in the CLDN5 and DGCR2 genes in the 22q11 deletion syndrome region and schizophrenia. Psychiatr Genet 18:255–256
Işık Ü, Aydoğan Avşar P, Aktepe E et al (2020) Serum zonulin and claudin-5 levels in children with obsessive–compulsive disorder. Nord J Psychiatry. https://doi.org/10.1080/08039488.2020.1715474
Julio-Pieper M, Bravo JA, Aliaga E et al (2014) Review article: intestinal barrier dysfunction and central nervous system disorders–a controversial association. Aliment Pharmacol Ther 40:1187–1201
Keaney J, Walsh DM, O’Malley T et al (2015) Autoregulated paracellular clearance of amyloid-β across the blood-brain barrier. Sci Adv 1:e1500472. https://doi.org/10.1126/sciadv.1500472
Kelly CP, Green PHR, Murray JA et al (2013) Larazotide acetate in patients with coeliac disease undergoing a gluten challenge: a randomised placebo-controlled study. Aliment Pharmacol Ther 37:252–262. https://doi.org/10.1111/apt.12147
Kılıç F, Işık Ü, Demirdaş A et al (2020) Serum zonulin and claudin-5 levels in patients with bipolar disorder. J Affect Disord 266:37–42. https://doi.org/10.1016/j.jad.2020.01.117
Leffler DA, Kelly CP, Abdallah HZ et al (2012) a randomized, double-blind study of larazotide acetate to prevent the activation of celiac disease during gluten challenge. Am J Gastroenterol 107:1554–1562. https://doi.org/10.1038/ajg.2012.211
Linninge C, Jönsson P, Bolinsson H et al (2018) Effects of acute stress provocation on cortisol levels, zonulin and inflammatory markers in low- and high-stressed men. Biol Psychol 138:48–55. https://doi.org/10.1016/J.BIOPSYCHO.2018.08.013
Maes M, Sirivichayakul S, Kanchanatawan B, Vodjani A (2019) Upregulation of the intestinal paracellular pathway with breakdown of tight and adherens junctions in deficit schizophrenia. Mol Neurobiol. https://doi.org/10.1007/s12035-019-1578-2
McDonald C, Murray RM (2000) Early and late environmental risk factors for schizophrenia. Brain Res Brain Res Rev 31(2–3):130–137. https://doi.org/10.1016/s0165-0173(99)00030-2
McGrath J, Saha S, Chant D, Welham J (2008) Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev 30:67–76. https://doi.org/10.1093/epirev/mxn001
Menard C, Pfau ML, Hodes GE et al (2017) Social stress induces neurovascular pathology promoting depression. Nat Neurosci 20:1752–1760. https://doi.org/10.1038/s41593-017-0010-3
Moreno-Navarrete JM, Sabater M, Ortega F et al (2012) Circulating zonulin, a marker of intestinal permeability, is increased in association with obesity-associated insulin resistance. PLoS ONE 7:e37160. https://doi.org/10.1371/journal.pone.0037160
Nishiura K, Ichikawa-Tomikawa N, Sugimoto K et al (2017) PKA activation and endothelial claudin-5 breakdown in the schizophrenic prefrontal cortex. Oncotarget 8:93382–93391. https://doi.org/10.18632/oncotarget.21850
Nitta T, Hata M, Gotoh S et al (2003) Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol 161:653–660. https://doi.org/10.1083/jcb.200302070
Omidinia E, Mashayekhi Mazar F, Shahamati P et al (2014) Polymorphism of the CLDN5 gene and schizophrenia in an Iranian population. Iran J Public Health 43:79–83
Özyurt G, Öztürk Y, Appak YÇ et al (2018) Increased zonulin is associated with hyperactivity and social dysfunctions in children with attention deficit hyperactivity disorder. Compr Psychiatry 87:138–142. https://doi.org/10.1016/j.comppsych.2018.10.006
Rahman MT, Ghosh C, Hossain M et al (2018) IFN-γ IL-17A, or zonulin rapidly increase the permeability of the blood-brain and small intestinal epithelial barriers: relevance for neuro-inflammatory diseases. Biochem Biophys Res Commun 507:274–279. https://doi.org/10.1016/j.bbrc.2018.11.021
Severance EG, Dickerson FB, Yolken RH (2018) Autoimmune phenotypes in schizophrenia reveal novel treatment targets. Pharmacol Ther 189:184–198. https://doi.org/10.1016/j.pharmthera.2018.05.005
Sun Z-Y, Wei J, Xie L et al (2004) The CLDN5 locus may be involved in the vulnerability to schizophrenia. Eur Psychiatry 19:354–357. https://doi.org/10.1016/j.eurpsy.2004.06.007
Tripathi A, Lammers KM, Goldblum S et al (2009) Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc Natl Acad Sci USA 106:16799–16804. https://doi.org/10.1073/pnas.0906773106
Tsukita S, Furuse M (2002) Claudin-based barrier in simple and stratified cellular sheets. Curr Opin Cell Biol 14:531–536. https://doi.org/10.1016/S0955-0674(02)00362-9
Turner JR (2009) Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 9:799–809. https://doi.org/10.1038/nri2653
Wei J, Hemmings GP (2005) Gene, gut and schizophrenia: the meeting point for the gene-environment interaction in developing schizophrenia. Med Hypotheses 64:547–552. https://doi.org/10.1016/j.mehy.2004.08.011
Wen L, Ley RE, Volchkov PY et al (2008) Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature 455:1109–1113. https://doi.org/10.1038/nature07336
Yücel M, Kotan D, Çiftçi GG et al (2016) Serum levels of endocan, claudin-5 and cytokines in migraine. Eur Rev Med Pharmacol Sci 20:930–936
Zhang D, Zhang L, Zheng Y et al (2014) Circulating zonulin levels in newly diagnosed Chinese type 2 diabetes patients. Diabetes Res Clin Pract 106:312–318. https://doi.org/10.1016/j.diabres.2014.08.017
Acknowledgements
We wish to thank all patients for participating in this study. We also wish to thank the Scientific Research Projects Coordination Unit of Süleyman Demirel University for their financial support (Number: TTU-2019-6946).
Funding
This study was funded by Scientific Research Projects Coordination Unit of Süleyman Demirel University (Number: TTU-2019-6946).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Andrea Schmitt.
Rights and permissions
About this article
Cite this article
Usta, A., Kılıç, F., Demirdaş, A. et al. Serum zonulin and claudin-5 levels in patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci 271, 767–773 (2021). https://doi.org/10.1007/s00406-020-01152-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-020-01152-9