Abstract
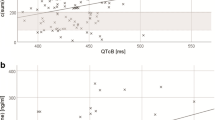
Despite safety concerns raised by the European Medicines Agency (EMA), evidence supporting QT-lengthening effects of escitalopram is far to be conclusive. We aimed to evaluate the relationship between escitalopram plasma levels (Escit-PL) and corrected QT-interval length (QTc-length) in 91 outpatients recruited from a hospital setting. Fifteen patients had an abnormally prolonged QTc-interval, and 3 had QTc-intervals ≥500 ms. No correlation between Escit-PL and QTc-length was found (r = 0.08; p = 0.45). Linear/logistic regression analyses were also conducted taking into account potential confounders such as age, gender, personal history of heart disease, medication load and concomitant use of antipsychotic/tricyclic antidepressants. Escit-PL did not predict either QTc-length or abnormally prolonged QTc-interval. Only antipsychotics/tricyclics use (adjusted β = 0.26, SE = 9.1; p = 0.01) was an independent predictor of QTc-length (R 2 = 0.096, F = 4.68, df = 2,88; p = 0.01). Only antipsychotics/tricyclics use (OR 3.56 [95% CI 1.01–12.52]; p < 0.05) and medication load (OR 1.32 [95% CI 1.06–1.64]; p < 0.01) were significantly associated with an increased risk of abnormally prolonged QTc-interval (Omnibus test χ 2 = 9.5, df = 2; p < 0.01). Our study did not find a significant relationship between Escit-PL and QTc-length even when recognized modulating factors of the QT-interval were controlled for. Concomitant use of other potentially arrhythmogenic agents may help to explain the apparent link between escitalopram and QT prolongation previously suggested. The advisability of maintaining the EMA warning is once again called into question.

Similar content being viewed by others
References
Beach SR, Celano CM, Noseworthy PA, Januzzi JL, Huffman JC (2013) Qtc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics 54:1–13
Leonard CE, Bilker WB, Newcomb C, Kimmel SE, Hennessy S (2011) Antidepressants and the risk of sudden cardiac death and ventricular arrhythmia. Pharmacoepidemiol Drug Saf 20:903–913
Sager PT (2008) Key clinical considerations for demonstrating the utility of preclinical models to predict clinical drug-induced torsades de pointes. Br J Pharmacol 154:1544–1549
Lin YL, Kung MF (2009) Magnitude of qt prolongation associated with a higher risk of torsades de pointes. Pharmacoepidemiol Drug Saf 18:235–239
Kobayashi T, Washiyama K, Ikeda K (2011) Inhibition of g protein-activated inwardly rectifying k+ channels by different classes of antidepressants. PLoS ONE 6:e28208
Kobayashi T, Washiyama K, Ikeda K (2004) Inhibition of g protein-activated inwardly rectifying k+ channels by various antidepressant drugs. Neuropsychopharmacology 29:1841–1851
Fda drug safety communication (2011) Abnormal heart rhythms associated with high doses of celexa (citalopram hydrobromide). http://www.fda.gov/drugs/drugsafety/ucm269086.htm. Accessed 15 May 2016
Bloch MH, McGuire J, Landeros-Weisenberger A, Leckman JF, Pittenger C (2010) Meta-analysis of the dose-response relationship of ssri in obsessive-compulsive disorder. Mol Psychiatry 15:850–855
Jakubovski E, Varigonda AL, Freemantle N, Taylor MJ, Bloch MH (2016) Systematic review and meta-analysis: dose-response relationship of selective serotonin reuptake inhibitors in major depressive disorder. Am J Psychiatry 173:174–183
Fda drug safety communication (2012) Celexa (citalopram hydrobromide) drug safety communication: Revised recommendations, potential risk of abnormal heart rhythms. Us food and drug administration. http://www.fda.Gov/drugs/drugsafety/ucm297391.htm. Accessed 15 May 2016
Forest laboratories (2016) Study cit-pk-15. Evaluation of the effects of sequential multiple-dose regimens of citalopram on cardiac repolarization in healthy subjects. http://www.forestclinicaltrials.com/ctrpdf.aspx?pdfid=cit-pk-15. Accessed 15 May 2016
Forest laboratories (2016) Study sct-pk-21. Evaluation of the effects of sequential multiple-dose regimens of escitalopram on cardiac repolarization in healthy subjects. http://www.forestclinicaltrials.com/ctrpdf.aspx?pdfid=sct-pk-21. Accessed 15 May 2016
European medicines agency (ema) (2011) Summary assessment report of the pharmacovigilance working party (phvwp). Escitalopram-risk of qt interval prolongation. http://www.ema.europa.eu/docs/en_gb/document_library/report/2011/11/wc500117988.pdf. Accessed 15 May 2016
European medicines agency (ema) (2011) Summary assessment report of the pharmacovigilance working party (phvwp). Citalopram-risk of qt interval prolongation. http://www.ema.europa.eu/docs/en_gb/document_library/report/2011/10/wc500117061.pdf. Accessed 15 May 2016
Alvarez E, Vieira S, Garcia-Moll X (2014) Citalopram, escitalopram and prolonged qt: warning or alarm? Rev Psiquiatr Salud Ment 7:147–150
Schwartz P, Altamura A (2012) Antidepressants, qt interval and cardiovascular risk. Justified concerns? J Psychopathol 18:183–191
Vieweg WV, Hasnain M, Howland RH, Hettema JM, Kogut C, Wood MA, Pandurangi AK (2012) Citalopram, qtc interval prolongation, and torsade de pointes. How should we apply the recent fda ruling? Am J Med 125:859–868
Kogut C, Crouse EB, Vieweg WV, Hasnain M, Baranchuk A, Digby GC, Koneru JN, Fernandez A, Deshmukh A, Hancox JC, Pandurangi AK (2013) Selective serotonin reuptake inhibitors and torsade de pointes: new concepts and new directions derived from a systematic review of case reports. Ther Adv Drug Saf 4:189–198
Hayes BD, Klein-Schwartz W, Clark RF, Muller AA, Miloradovich JE (2010) Comparison of toxicity of acute overdoses with citalopram and escitalopram. J Emerg Med 39:44–48
Waring WS, Graham A, Gray J, Wilson AD, Howell C, Bateman DN (2010) Evaluation of a qt nomogram for risk assessment after antidepressant overdose. Br J Clin Pharmacol 70:881–885
Beach SR, Kostis WJ, Celano CM, Januzzi JL, Ruskin JN, Noseworthy PA, Huffman JC (2014) Meta-analysis of selective serotonin reuptake inhibitor-associated qtc prolongation. J Clin Psychiatry 75:E441–E449
Castro VM, Clements CC, Murphy SN, Gainer VS, Fava M, Weilburg JB, Erb JL, Churchill SE, Kohane IS, Iosifescu DV, Smoller JW, Perlis RH (2013) Qt interval and antidepressant use: a cross sectional study of electronic health records. BMJ 346:f288
Rasmussen SL, Overo KF, Tanghoj P (1999) Cardiac safety of citalopram: prospective trials and retrospective analyses. J Clin Psychopharmacol 19:407–415
Thase ME, Larsen KG, Reines E, Kennedy SH (2013) The cardiovascular safety profile of escitalopram. Eur Neuropsychopharmacol 23:1391–1400
van Haelst IM, van Klei WA, Doodeman HJ, Warnier MJ, De Bruin ML, Kalkman CJ, Egberts TC (2014) Qt interval prolongation in users of selective serotonin reuptake inhibitors in an elderly surgical population: a cross-sectional study. J Clin Psychiatry 75:15–21
Ji Y, Schaid DJ, Desta Z, Kubo M, Batzler AJ, Snyder K, Mushiroda T, Kamatani N, Ogburn E, Hall-Flavin D, Flockhart D, Nakamura Y, Mrazek DA, Weinshilboum RM (2014) Citalopram and escitalopram plasma drug and metabolite concentrations: genome-wide associations. Br J Clin Pharmacol 78:373–383
Hiemke C (2008) Therapeutic drug monitoring in neuropsychopharmacology: does it hold its promises? Eur Arch Psychiatry Clin Neurosci 258(Suppl 1):21–27
van Noord C, Straus SM, Sturkenboom MC, Hofman A, Aarnoudse AJ, Bagnardi V, Kors JA, Newton-Cheh C, Witteman JC, Stricker BH (2009) Psychotropic drugs associated with corrected qt interval prolongation. J Clin Psychopharmacol 29:9–15
Woosley, rl and romero, ka (2016). Qtdrugs list, azcert, inc. 1822 innovation park dr., oro valley, az 85755. http://www.crediblemeds.org. Accessed 15 May 2016
Taylor DM (2003) Antipsychotics and qt prolongation. Acta Psychiatr Scand 107:85–95
Almeida JRC, Akkal D, Hassel S, Travis MJ, Banihashemi L, Kerr N, Kupfer DJ, Phillips ML (2009) Reduced gray matter volume in ventral prefrontal cortex but not amygdala in bipolar disorder: significant effects of gender and trait anxiety. Psychiatry Res Neuroimaging 171:54–68
de Diego-Adeliño J, Pires P, Gómez-Ansón B, Serra-Blasco M, Vives-Gilabert Y, Puigdemont D, Martín-Blanco A, Alvarez E, Pérez V, Portella MJ (2014) Microstructural white-matter abnormalities associated with treatment resistance, severity and duration of illness in major depression. Psychol Med 44:1171–1182
Forrester MB (2007) Escitalopram ingestions reported to texas poison control centers, 2002–2005. Hum Exp Toxicol 26:473–482
Meyboom RH, Hekster YA, Egberts AC, Gribnau FW, Edwards IR (1997) Causal or casual? The role of causality assessment in pharmacovigilance. Drug Saf 17:374–389
Girardin FR, Gex-Fabry M, Berney P, Shah D, Gaspoz JM, Dayer P (2013) Drug-induced long qt in adult psychiatric inpatients: the 5-year cross-sectional ecg screening outcome in psychiatry study. Am J Psychiatry 170:1468–1476
Justo D, Prokhorov V, Heller K, Zeltser D (2005) Torsade de pointes induced by psychotropic drugs and the prevalence of its risk factors. Acta Psychiatr Scand 111:171–176
Tseng PT, Lee Y, Lin YE, Lin PY (2012) Low-dose escitalopram for 2 days associated with corrected qt interval prolongation in a middle-aged woman: a case report and literature review. Gen Hosp Psychiatry 34:210.e213-215
Friedman MJ, Mull CC, Sharieff GQ, Tsarouhas N (2003) Prolonged qt syndrome in children: an uncommon but potentially fatal entity. J Emerg Med 24:173–179
Hanash JA, Hansen BH, Hansen JF, Nielsen OW, Rasmussen A, Birket-Smith M (2012) Cardiovascular safety of one-year escitalopram therapy in clinically nondepressed patients with acute coronary syndrome: results from the depression in patients with coronary artery disease (decard) trial. J Cardiovasc Pharmacol 60:397–405
Kim JM, Bae KY, Stewart R, Jung BO, Kang HJ, Kim SW, Shin IS, Hong YJ, Kim JH, Shin HY, Kang G, Ahn Y, Kim JK, Jeong MH, Yoon JS (2015) Escitalopram treatment for depressive disorder following acute coronary syndrome: a 24-week double-blind, placebo-controlled trial. J Clin Psychiatry 76:62–68
Lindsley CW (2012) The top prescription drugs of 2011 in the united states: antipsychotics and antidepressants once again lead cns therapeutics. ACS Chem Neurosci 3:630–631
Libby AM, Orton HD, Valuck RJ (2009) Persisting decline in depression treatment after fda warnings. Arch Gen Psychiatry 66:633–639
Cipriani A, Santilli C, Furukawa TA, Signoretti A, Nakagawa A, McGuire H, Churchill R, Barbui C (2009) Escitalopram versus other antidepressive agents for depression. Cochrane Database Syst Rev:CD006532
Ramsberg J, Asseburg C, Henriksson M (2012) Effectiveness and cost-effectiveness of antidepressants in primary care: a multiple treatment comparison meta-analysis and cost-effectiveness model. PLoS ONE 7:e42003
Acknowledgements
Dr. de Diego-Adeliño is funded by the Instituto de Salud Carlos III through a “Juan Rodés” research fellowship (JR14/00011). Dr. Portella is funded by the Ministerio de Ciencia e Innovación of the Spanish Government and by the Instituto de Salud Carlos III through a “Miguel Servet” research contract (CP10-00393), co-financed by the European Regional Development Fund (ERDF) (2013–2016). The funding agencies mentioned had no role in the design, conduct and publication of the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. de Diego-Adeliño has received lecture honoraria from Pfizer, GlaxoSmithKline and Lundbeck. Dr. Alvarez has received consulting and lecture honoraria from Eli Lilly, Lundbeck, Pfizer, Sanofi-Aventis and Otsuka; has participated as principal local investigator in clinical trials sponsored by Eli Lilly, Bristol-Myrers Squibb, Sanofi-Aventis and AB·Biotics; and has served as national coordinator of clinical trials sponsored by Servier and Lundbeck. The remaining authors report no financial or other relationship relevant to the subject of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Carceller-Sindreu, M., de Diego-Adeliño, J., Portella, M.J. et al. Lack of relationship between plasma levels of escitalopram and QTc-interval length. Eur Arch Psychiatry Clin Neurosci 267, 815–822 (2017). https://doi.org/10.1007/s00406-016-0758-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-016-0758-6