Abstract
Purpose
To investigate the rate of residual disease in the Potsic staging system for congenital cholesteatomas.
Methods
A protocol registration was published on PROSPERO (CRD42022383932), describing residual disease as a primary outcome and hearing improvement as secondary. A systematic search was performed in four databases (PubMed, Embase, Cochrane Library, Web of Science) on December 14, 2022. Articles were included if cholesteatomas were staged according to the Potsic system and follow-up duration was documented. Risk of bias was evaluated using the Quality In Prognosis Studies (QUIPS) tool. In the statistical synthesis a random effects model was used. Between-study heterogeneity was assessed using I2.
Results
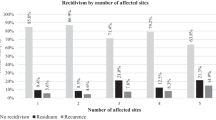
Thirteen articles were found to be eligible for systematic review and seven were included in the meta-analysis section. All records were retrospective cohort studies with high risk of bias. Regarding the proportions of residual disease, analysis using the χ2 test showed no statistically significant difference between Potsic stages after a follow-up of minimum one year (stage I 0.06 (confidence interval (CI) 0.01–0.33); stage II 0.20 (CI 0.09–0.38); stage III 0.06 (CI 0.00–0.61); stage IV: 0.17 (CI 0.01–0.81)). Postoperative and preoperative hearing outcomes could not be analyzed due to varied reporting. Results on cholesteatoma location and mean age at staging were consistent with those previously published.
Conclusion
No statistically significant difference was found in the proportions of residual disease between Potsic stages, thus the staging system’s applicability for outcome prediction could not be proven based on the available data. Targeted studies are needed for a higher level of evidence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The most widely used staging system for congenital cholesteatoma is the one that was described by Potsic and colleagues in 2002 [1], which is based on the extension of the disease behind an intact tympanic membrane. A cholesteatoma limited to a single tympanic membrane quadrant is referred to as stage I, while a cholesteatoma mass behind two or more quadrants without ossicular chain involvement or mastoid extension is called stage II. Stage III means that the ossicular chain is affected but without mastoid involvement, and the cholesteatoma enters the mastoid space in stage IV disease.
There are numerous advantages to adopting a staging system in cholesteatoma management. Ideally, it could allow for more precise patient counseling about disease severity and the likelihood of experiencing residual cholesteatoma, help comparing treatment results on a multi-institutional level, choose proper surgical approach, and assess the need for second-look surgery.
As congenital cholesteatoma is an uncommon disease, accounting for only 1–5% [2] of all cholesteatomas, mostly smaller case series were published in the literature, with some of them using the Potsic staging system to categorize patients [3]. As it was described when introduced, one of the dedicated goals of this staging system is outcome prediction [1]. However, the real prognostic value of this system in estimating the occurrence of residual disease remains uncertain. To obtain a higher level of evidence on the usefulness of the Potsic staging system, a joint data analysis of these studies is needed. Therefore, we aimed to assess whether the Potsic staging system was indeed indicative of the rate of residual disease and whether the extent of hearing improvement was also connected to the stage at presentation.
Methods
The following systematic review and meta-analysis was based on recommendations of the PRISMA 2020 guideline [4] and the Cochrane Handbook [5]. The protocol of the study was created using a PICO framework [6], has been registered on PROSPERO (registration number: CRD42022383932) and was adhered to in every step.
Inclusion and exclusion criteria
We included patients of any age and sex, diagnosed with congenital cholesteatoma (with or without symptoms) based on the Levenson criteria [7]. These criteria are the following: upon examination a pearly white mass can be observed medial to an intact tympanic membrane with a normal pars tensa and flaccida and no history of tympanic membrane perforation, no previous otologic procedures in the anamnesis, but previous occurrences of otitis media are allowed. Additional criteria for selected studies were that all patients were undergoing primary surgery, and afterwards followed up for more than 12 months, and their cholesteatoma was graded using the Potsic staging system. For the records selected for the systematic review section of our report we also included sources with a follow-up period shorter than 12 months to allow for a wider scope of publications on this rare entity that we studied.
We included published retrospective cohort studies and observational studies written in English, whereas conference abstracts, guidelines, systematic reviews of literature, meta-analyses, animal studies, case reports, case series and reports predating the publication of the Potsic staging system were excluded. Reports that included no information based on residual disease in each stage were likewise excluded, together with patients whose outcome has been individually reported but were lost to follow-up within one year of surgery.
Information sources and search strategy
A systematic search was performed on December 14, 2022, in PubMed, Embase, Web of Science, and Cochrane Library using the following search key, without applying filters:
(“Potsic” and stag* or system or classification or congenital or pediatric) AND cholesteatoma.
Reference lists of all identified studies found eligible during text selection were reviewed for additional reports using CitationChaser [8].
Selection process
After automatic and manual removal of duplicates using a citation manager program (EndNote 20, Clarivate Analytics, Philadelphia, PA, USA), title, abstract and full-text selection was performed by two independent review authors (BK and KSH) using the management program Rayyan [9]. After each selection step disagreements were resolved by a third reviewer (TH) and inter-rater reliability was measured with Cohen’s kappa calculation [10].
Data collection process
The aforementioned authors (BK and KSH) independently completed the data extraction using a predesigned Excel (Microsoft Corporation, Redmond, Washington, United States) datasheet. The following data were extracted from each eligible article if available: name of the first author, year of publication, basic demographic characteristics (proportion of females, age, number of patients), number of patients assigned to each stage, the number of residual cases in these stages, surgery technique, length of follow-up period mean, median, standard deviation and range measured in months, hearing outcomes, type and location of the cholesteatoma, exclusion and inclusion criteria. Disagreements on data extraction were resolved by discussion between the authors.
Data items
Our main outcome was the proportion of residual disease (defined as keratinizing squamous epithelium left behind in the tympanic or mastoid cavity serving as a focal point for new cholesteatoma growth) [11] in each Potsic stage. Secondary outcomes were preoperative cholesteatoma location behind the quadrants of the tympanic membrane, improvement in hearing measured by preoperative and postoperative pure tone average or air bone gap, and mean age at diagnosis. Studies were ineligible if these outcomes were either not measured or measured but not reported.
Study risk of bias assessment
The two authors (KBK and KSH) performed the risk of bias assessment and visualized the results independently using the QUIPS tool [12]. Disagreements arising in the process were resolved through discussion.
Effect measures and synthesis methods
To calculate the proportions, in each Potsic stage we used the total number of patients and those with residual diseases to measure effect size. We then used a random intercept meta-logistic regression model to pool proportions where the stage was a moderator variable [13]. Knapp–Hartung adjustments [14] were applied to the parameter estimates to control for the uncertainty in between-study heterogeneity. We used the maximum likelihood method to estimate the random effect heterogeneity (τ2). For the different stages where we assumed that subgroups had different τ2 values as we anticipated differences in the between-study heterogeneity in the subgroups. We assessed the difference between the subgroups with the Cochrane Q test [13]. I2 was used to assess the between-study heterogeneity following the recommendations of Harrer et al. [13].
Reporting bias assessment
Funnel plots were applied to report and visualize publication bias.
Certainty assessment
Level of evidence was ranked using the Oxford Centre of Evidence Based Medicine (OCEBM) 2011 Levels of Evidence table [15], assisted by the OCEBM Background document [16].
Results
Study selection
Our selection process is detailed in a PRISMA flowchart pictured in Fig. 1. Inter-rater reliability according to Cohen’s kappa was 0.94 for title and abstract selection, and 1.00 for full-text selection. We have found 13 publications [17,18,19,20,21,22,23,24,25,26,27,28,29] that satisfied our criteria, others were excluded because they used no staging system or one different from the one proposed by Potsic et al. [1]. Reference lists of included full texts yielded no further eligible reports.
Results and characteristics of individual studies
In our systematic review, all the included studies (their characteristics are listed in Table 1) were retrospective cohort studies, based on reviewing charts of patients previously treated for congenital cholesteatoma, diagnosed according to the criteria proposed by Levenson et al. [7].
Due to the low incidence rate of congenital cholesteatoma, only one publication [21] included more than 100 patients. The total number of patients included in our review was 633, of whom 216 were female, their ages spanning between six months and 37 years.
A wide range of outcomes was documented apart from our primary and secondary outcomes, such as the incidence of postoperative complications, morphological findings, and the rate of open- and closed-type cholesteatoma.
Risk of bias in studies (Supplementary material, Table 1)
As a result of assessing the risk of bias, all key articles proved to have high risk of bias according to the QUIPS tool [12]. Reasons were insufficient reporting on patients lost to follow-up, not establishing how patients were staged and whether this was done in a standardized way, and failing to define or measure potential confounders.
Results of syntheses
The primary synthesis described in our protocol assessed whether Potsic stage at presentation was predictive of residual disease. The secondary outcome was hearing improvement and additional, not prespecified outcomes were cholesteatoma location and mean age at diagnosis.
Results of statistical synthesis
The forest plot in Fig. 2, which details the results of our meta-analysis, shows the proportion of residual disease in each stage, based on data from 259 patients with a total of 33 residual cases, divided into four stages. Seven reports [17, 18, 20, 22,23,24, 28] were included in this analysis, where follow-up time of all patients or single patients whose individual data was published within the report exceeded 12 months after surgery.
Results of the statistical analysis showed wide confidence intervals (CI) (CI 0.01–0.33 in stage I; CI 0.09–0.38 in stage II; CI 0.00–0.61 in stage III; CI 0.01–0.81 in stage IV) and high p values (1.00 in stage I; 0.99 in stage II; 1.00 in stage III and 0.28 in stage IV) with I2 values (0% in stage I–III and 22% in stage IV, overall 0%), suggesting that exact heterogeneity was hard to assess. The intergroup difference was tested with χ23 test (χ23 = 3.17 p = 0.37). Thus, differences in the proportions of patients with residual disease in each stage were not shown to be statistically significant.
Results of qualitative analyses
Location of cholesteatoma
Location of cholesteatoma was detailed in ten reports [20, 22,23,24,25,26, 28, 29]. Figure 3 shows our graph summarizing the occurrences of cholesteatoma behind each quadrant of the tympanic membrane, in the attic, and in the mastoid space. In cases where two or more locations were specified, rather than pairing them in separate groups, both were added to their significant cluster.
As a result, we have found that the anterosuperior quadrant was the most common site for cholesteatomas (214 of 563 affected quadrants), followed by the posterosuperior quadrant (163). Less common locations are the posteroinferior quadrant (45), anterior quadrants [20, 25] (not specified) (35), posterior quadrants (not specified) (29), anteroinferior quadrant (29), atticus or epitympanic space [18] (15), mastoid cavity (9), superior quadrants (not specified) (2) and central quadrant [20] (not specified) (1). In 22 cases, multiple [17, 23, 26, 29] (not specified) quadrants were reported.
Hearing improvement
As shown in Table 2, hearing results were reported in nine studies [17,18,19,20,21,22,23,24,25, 27,28,29]. All of them used a slightly different approach to assessing hearing improvement, and none of them followed the guidelines of the Committee on Hearing and Equilibrium [30]. Although analyzing hearing improvement was registered as our secondary outcome, we were unable to pool the published results.
One study [29] showed a decreased rate of hearing improvement in more advanced stages. Similarly, in two other reports [18, 27], a significant relationship was shown between stage and postoperative pure tone average (PTA). Although no quantitative analysis was performed, results of a third publication [19] also show that pre- and post-operative hearing results were worse among stage III patients. The remaining two publications either found no significant postoperative hearing improvement [25] or showed no deterioration based on the results available for seven patients [20, 22].
Mean age at staging
Eight studies [17, 19, 20, 22,23,24, 26, 29] out of our 13 key articles had information on age at diagnosis or surgery. In three reports [19, 22, 23], age had been specified as age at first surgery. Five articles [17, 20, 22,23,24] had individual patient data, and three [19, 26, 29] had staged means available.
To be able to calculate an approximate mean from these three articles, we have summarized (Fig. 4) the ages of patients by multiplying the mean age by the number of patients in each stage. Thus, the approximate mean age at staging came to be 3.5 years for stage I, 4.3 years for stage II, 7.5 years for stage III, and 9.4 years for stage IV based on data from 129, 53, 90, and 35 patients, respectively. The data exhibits a trend of increasing patient age as the disease becomes more extensive.
Analysis of publication bias
Publication bias was visualized with contour enhanced funnel plots, presented in Fig. 1 of the Supplementary material. No statistical testing was conducted (as less than 10 studies were included) per the recommendations of Harrer et al. [13].
Certainty analysis
The overall level of certainty could not be assessed according to OCEBM 2011[15] because this tool is not suitable for retrospective cohort studies, which was study design used exclusively in our key articles.
Discussion
To summarize our results, in our meta-analysis of seven reports [17, 18, 20, 22,23,24, 28] involving 259 patients, we were unable to detect a statistically significant difference in the proportion of residual disease between the four Potsic stages, based on data from patients who were followed for at least 12 months after cholesteatoma removal. Hearing improvement could not be assessed due to varied reporting. Cholesteatomas were most frequently discovered behind the anterosuperior quadrant of the tympanic membrane, and there was a distinct trend of more advanced disease in older patients. Both outcomes were consistent with already existing results in the literature.
In their landmark study [1], Potsic and colleagues described some important criteria that an ideal staging system should meet: the categories should be simple and unambiguous with commensurable patient cohort sizes in each stage, and the staging system should also have good predictive value. From the perspective of our patients, the most important of these aspects is outcome prediction. The popularity of this staging system indicates that most of these expectations seem to be met. Although low incidence of this disease means that studies have usually included less than 100 patients, credible confirmation of the stage-based prediction needs much larger sample sizes.
This led us to perform a meta-analysis, collecting all the studies where the reported outcome was connected to the Potsic stage. Additional desired outcomes were to determine an ideal length of follow-up and assess the need for staged surgery and reexploration in early-stage disease. The clinical implication for a reliable staging system is to minimize time spent in the hospital for young children and reduce the number of operations performed on their ears by choosing the proper operation technique as a first procedure, thus optimizing surgical outcomes.
We have found no previous meta-analyses investigating the association between the rate of residual disease and Potsic stage, but several of our key articles [18, 19, 27] have found an association between advanced-stage cholesteatoma and an increased likelihood of residual disease. One article [26] found no association between stage and residual disease, and another [20] reported that the staging system does not reflect the differences between anteriorly and posteriorly located cholesteatoma.
Several patients from those included in our key articles [23, 24, 28] presented with residual disease after one year, which suggests that a follow-up period longer than 12 months would be desirable. However, in most articles that we found during our search process, some patients were lost to follow-up much sooner. In this analysis we had no choice but to exclude them to provide an optimal level of evidence, which could have been further enhanced, if more data on these patients had been available.
For our secondary outcome of hearing improvement, we planned to conduct a subgroup analysis to compare the stages. Although a guideline has already been published for reporting audiology test results [30], the nine articles [18,19,20, 22, 24,25,26,27, 29] in our analysis that did report on audiological results of all or some studies used different measurements (different frequency thresholds, ABG or PTA based on air conduction, bone conduction or both), which rendered their results unpoolable.
Location of cholesteatoma has been most frequently reported [17, 31,32,33] to be seen behind the anterosuperior quadrant, however Inokuchi et al. [18] found that patients in East Asia more frequently present with more extensive cholesteatomas involving multiple subregions compared to patients included in other analyses. Our report also found this quadrant to be the one most often affected.
Increase of cholesteatoma size had previously been linked to older age [34], indicating the expansive nature of cholesteatoma. In our analysis, we have also found a connection between advanced disease and older age.
We must highlight that all the included studies had a high risk of bias. As there are no dedicated tools available for reports on staging systems, we have decided to use QUIPS [12] to assess risk of bias, because this tool fit our study design best. However, there were several criteria of QUIPS that the included studies typically failed to meet. In these domains lack of data and deficient reporting resulted in a high risk of bias. Two domains (domain 2 and 4) of the QUIPS tool focuses on whether the reason for patients dropping out of follow-up was listed (together with attempts to collect information from them) and whether the exact duration of follow-up was described in the article. Domain 4 states that a clear definition should be given for the outcomes (in our case incidence of residual disease and extent of hearing improvement), and these are ought to be measured in the same way for all patients. In domain 5, possible confounding factors are an important point, such as different surgical techniques, which have been previously shown to potentially influence the likelihood of cholesteatoma recidivism [35]. In the future, including such data in reports is essential to generate high-level evidence.
Strengths and limitations
Our analysis is the first comprehensive project to summarize the results of congenital cholesteatoma surgery after considerable follow-up (minimum 1 year) and to process them according to the most widely used staging system for this disease. It is also the first to examine the effectiveness of the staging system in predicting outcome, which, according to its authors is a vital feature of a good staging system. During our analysis, we have applied rigorous methodology and followed international guidelines for summarizing previously published data.
Limitations include the retrospective nature of the key articles, which led to heterogeneous surgical techniques, varied measurement of hearing outcomes, and inadequate data on follow-up and attrition. The rarity of congenital cholesteatoma resulted in small patient cohorts and few reports. Other limitations are pooled data in articles (e.g., for mean age at staging we can only calculate estimates) and diverse duration of follow-up periods, which prevented us from including hazard ratios into out forest plot and calculating the exact desirable follow-up duration, wherein residual disease can reasonably be expected.
Implication for practice and research
Should more individual data documenting longer follow-up periods become available, it would be even more informative to calculate hazard ratios for each stage. However, at this time, based on the currently available data in the published literature this was not possible. In the future, more cases with carefully documented, long spanning individual follow-up data (especially in reports with small case numbers) are needed for a proper assessment. Durations of follow-up and the reason for losing patients in the process should also be documented.
In a clinical setting, second-look surgery should not be omitted purely based on stage; however, since the staging system’s abilities seem more promising for other outcomes, it is still preferable to stage cholesteatomas using this system.
Conclusion
In our systematic review and meta-analysis, we found that although the staging system published by Potsic et al. theoretically meets the authors’ first three criteria for a good staging system, based on data published so far, we cannot confirm its prognostic value. Therefore, prospective, well-designed studies are necessary with rigorously documented follow-up to determine this staging system’s true ability to predict occurrence of residual disease.
Data availability
The datasets used in this study can be found in the full-text articles included in the systematic review and meta-analysis.
References
Potsic WP, Samadi DS, Marsh RR, Wetmore RF (2002) A staging system for congenital cholesteatoma. Arch Otolaryngol Head Neck Surg 128:1009–1012. https://doi.org/10.1001/archotol.128.9.1009
Bennett M, Warren F, Jackson GC, Kaylie D (2006) Congenital cholesteatoma: theories, facts, and 53 patients. Otolaryngol Clin North Am 39:1081–1094
Gilberto N, Custódio S, Colaço T, Santos R, Sousa P, Escada P (2020) Middle ear congenital cholesteatoma: systematic review, meta-analysis and insights on its pathogenesis. Eur Arch Otorhinolaryngol 277:987–998. https://doi.org/10.1007/s00405-020-05792-4
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The prisma 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Higgins JPT, TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (2022) Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022). Cochrane Database of Systematic Reviews
Huang X, Lin J, Demner-Fushman D (2006) Evaluation of pico as a knowledge representation for clinical questions. AMIA Annu Symp Proc 2006:359–363
Levenson MJ, Parisier SC, Chute P, Wenig S, Juarbe C (1986) A review of twenty congenital cholesteatomas of the middle ear in children. Otolaryngol Head Neck Surg 94:560–567. https://doi.org/10.1177/019459988609400505
Haddaway NR, Grainger MJ, Gray CT (2022) Citationchaser: a tool for transparent and efficient forward and backward citation chasing in systematic searching. Res Synth Methods. 13:533–545. https://doi.org/10.1002/jrsm.1563
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A (2016) Rayyan-a web and mobile app for systematic reviews. Syst Rev 5:210. https://doi.org/10.1186/s13643-016-0384-4
McHugh ML (2012) Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 22:276–282
Yung M, Tono T, Olszewska E, Yamamoto Y, Sudhoff H, Sakagami M, Mulder J, Kojima H, İncesulu A, Trabalzini F, Özgirgin N (2017) Eaono/jos joint consensus statements on the definitions, classification and staging of middle ear cholesteatoma. J Int Adv Otol 13:1–8. https://doi.org/10.5152/iao.2017.3363
Grooten WJA, Tseli E, Äng BO, Boersma K, Stålnacke B-M, Gerdle B, Enthoven P (2019) Elaborating on the assessment of the risk of bias in prognostic studies in pain rehabilitation using quips—aspects of interrater agreement. Diagn Progn Res 3:5. https://doi.org/10.1186/s41512-019-0050-0
Harrer M, Cuijpers P, Furukawa Toshi A, Ebert DD (2021) Doing meta-analysis with r: a hands-on guide. Chapman & Hall/CRC Press, London, Boca Raton
Knapp G, Hartung J (2003) Improved tests for a random effects meta-regression with a single covariate. Stat Med 22:2693–2710. https://doi.org/10.1002/sim.1482
OCEBM Levels of Evidence Working Group (Jeremy Howick ICJLL, Paul Glasziou, Trish Greenhalgh, Carl Heneghan, Alessandro Liberati, Ivan Moschetti, Bob Phillips, Hazel Thornton, Olive Goddard and Mary Hodgkinson) “The oxford levels of evidence 2”. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence. Accessed 2023.01.27.
Jeremy Howick IC, Paul Glasziou, Trish Greenhalgh, Carl Heneghan, Alessandro Liberati, Ivan Moschetti, Bob Phillips, and Hazel Thornton (2011) Explanation of the 2011 oxford centre for evidence-based medicine (ocebm) levels of evidence (background document). https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence. Accessed 2023.02.22.
Cho HS, Kim HG, Jung DJ, Jang JH, Lee SH, Lee KY (2016) Clinical aspects and surgical outcomes of congenital cholesteatoma in 93 children: Increasing trends of congenital cholesteatoma from 1997 through 2012. J Audiol Otol 20:168–173. https://doi.org/10.7874/jao.2016.20.3.168
Inokuchi G, Okuno T, Hata Y, Baba M, Sugiyama D (2010) Congenital cholesteatoma: posterior lesions and the staging system. Ann Otol Rhinol Laryngol 119:490–494. https://doi.org/10.1177/000348941011900711
Jenks CM, Purcell PL, Federici G, Villari D, Presutti L, James AL, Hoff SR (2022) Transcanal endoscopic ear surgery for congenital cholesteatoma: a multi-institutional series. Otolaryngol Head Neck Surg 167:537–544. https://doi.org/10.1177/01945998211067502
Kim BJ, Kim JH, Park MK, Lee JH, Oh SH, Suh MW (2018) Endoscopic visualization to the anterior surface of the malleus and tensor tympani tendon in congenital cholesteatoma. Eur Arch Otorhinolaryngol 275:1069–1075. https://doi.org/10.1007/s00405-018-4917-4
Kim H, Yoo SY, Choung YH, Park HY (2019) Is transcanal tympanoplasty an appropriate surgical treatment for congenital middle ear cholesteatoma with ossicular involvement? Int J Pediatr Otorhinolaryngol 116:102–106. https://doi.org/10.1016/j.ijporl.2018.10.030
Kobayashi T, Gyo K, Komori M, Hyodo M (2015) Efficacy and safety of transcanal endoscopic ear surgery for congenital cholesteatomas: a preliminary report. Otol Neurotol 36:1644–1650. https://doi.org/10.1097/mao.0000000000000857
Lee SH, Jang JH, Lee D, Lee HR, Lee KY (2014) Surgical outcomes of early congenital cholesteatoma: minimally invasive transcanal approach. Laryngoscope 124:755–759. https://doi.org/10.1002/lary.24313
Park JH, Ahn J, Moon IJ (2018) Transcanal endoscopic ear surgery for congenital cholesteatoma. Clin Exp Otorhinolaryngol 11:233–241. https://doi.org/10.21053/ceo.2018.00122
Park KH, Park SN, Chang KH, Jung MK, Yeo SW (2009) Congenital middle ear cholesteatoma in children; retrospective review of 35 cases. J Korean Med Sci 24:126–131
Song IS, Han WG, Lim KH, Nam KJ, Yoo MH, Rah YC, Choi J (2019) Clinical characteristics and treatment outcomes of congenital cholesteatoma. J Int Adv Otol 15:386–390. https://doi.org/10.5152/iao.2019.6279
Stapleton AL, Egloff AM, Yellon RF (2012) Congenital cholesteatoma: Predictors for residual disease and hearing outcomes. Arch Otolaryngol Head Neck Surg 138:280–285. https://doi.org/10.1001/archoto.2011.1422
Takagi T, Gyo K, Hakuba N, Hyodo J, Hato N (2014) Clinical features, presenting symptoms, and surgical results of congenital cholesteatoma based on Potsic’s staging system. Acta Otolaryngol 134:462–467. https://doi.org/10.3109/00016489.2013.875218
Yamatodani T, Mizuta K, Hosokawa K, Takizawa Y, Sugiyama K, Nakanishi H, Mineta H (2013) Congenital middle ear cholesteatoma: experience from 26 surgical cases. Ann Otol, Rhinol Laryngol 122:316–321. https://doi.org/10.1177/000348941312200505
Edwin M, Monsell M, Balkany TA, Gates GA, Goldenberg RA, Meyerhoff WL, House JW (1995) Committee on hearing and equilibrium guidelines for the evaluation of results of treatment of conductive hearing loss. American academy of otolaryngology-head and neck surgery foundation, inc. Otolaryngol Head Neck Surg 113:186–187. https://doi.org/10.1016/s0194-5998(95)70103-6
Potsic WP, Korman SB, Samadi DS, Wetmore RF (2002) Congenital cholesteatoma: 20 years’ experience at the children’s hospital of Philadelphia. Otolaryngol Head Neck Surg 126:409–414. https://doi.org/10.1067/mhn.2002.123446
Nelson JD (1988) Chronic suppurative otitis media. Pediatr Infect Dis J 7:446–448. https://doi.org/10.1097/00006454-198806000-00033
Nelson M, Roger G, Koltai PJ, Garabedian EN, Triglia JM, Roman S, Castellon RJ, Hammel JP (2002) Congenital cholesteatoma: classification, management, and outcome. Arch Otolaryngol Head Neck Surg 128:810–814. https://doi.org/10.1001/archotol.128.7.810
Lim HW, Yoon TH, Kang WS (2012) Congenital cholesteatoma: clinical features and growth patterns. Am J Otolaryngol 33:538–542. https://doi.org/10.1016/j.amjoto.2012.01.001
Illés K, Meznerics FA, Dembrovszky F, Fehérvári P, Bánvölgyi A, Csupor D, Hegyi P, Horváth T (2023) Mastoid obliteration decreases the recurrent and residual disease: Systematic review and meta-analysis. Laryngoscope 133:1297–1305. https://doi.org/10.1002/lary.30413
Funding
Open access funding provided by Semmelweis University. No funds, grants, or other support was received to assist in the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
BK: conceptualization, project administration, data curation, formal analysis, writing-original draft; KS-H: methodology; PH: funding acquisition, methodology; ZM: methodology; AS-W: methodology; PF: data curation, formal analysis; AH: conceptualization; KI: conceptualization, methodology, supervision; writing-review and editing; TH: conceptualization, supervision, writing-review and editing. All authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing non- or financial interests to declare.
Ethical approval
No ethical approval or consent was required for this systematic review with meta-analysis, as all data were already published in peer-reviewed journals.
Patient consent
No patients were involved in the present study’s design, conduct, or interpretation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
405_2024_8478_MOESM1_ESM.docx
Supplementary file1 Supplementary material figure 1. Contrast enhanced funnel plot to visualize publication bias. Supplementary material figure 2. Visualization of the risk of bias assessment using the QUIPS tool. (DOCX 1129 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Körmendy, K.B., Shenker-Horváth, K., Shulze Wenning, A. et al. Predicting residual cholesteatoma with the Potsic staging system still lacks evidence: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol (2024). https://doi.org/10.1007/s00405-024-08478-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00405-024-08478-3