Abstract
Introduction
COVID-19 can result in an extensive range of extrapulmonary, and neurological signs and symptoms such as olfactory and/or taste dysfunction, and otologic symptoms. The aim of this study was to investigate the hearing loss manifestation from COVID-19.
Methods
The goal of this umbrella review was to examine hearing loss associated with COVID-19 disease. English literature published until October 15, 2022 in online databases including PubMed, Scopus, Web of Science, and Embase was considered for this purpose. Eligibility of the articles for subsequent data extraction was evaluated in a two-step selection process with consideration to an inclusion/exclusion criterion. This review followed the PRISMA protocol and the Amstar-2 checklist for quality assessment.
Results
A total of four treatment strategies were used by different studies which included oral corticosteroids, intratympanic corticosteroids, combined oral and intratympanic corticosteroids, and hyperbaric oxygen therapy. Five studies investigated corticosteroid use in the forms of oral or intratympanic injection; four studies reported (complete or partial) hearing improvements after steroid treatment, while one study stated no significant improvement in hearing function. One study reported that oral corticosteroid monotherapy alone was not effective, while vestibular symptoms were ameliorated by a combination of oral prednisone, intratympanic dexamethasone injection, and hydroxychloroquine.
Conclusion
The findings suggest that despite being one of the rare complications of COVID-19, hearing loss can impact a patient's quality of life. The most common type reported was sensorineural hearing loss, which can be diagnosed with variable techniques.
Similar content being viewed by others
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the cause of novel coronavirus disease-2019 (COVID-19), first emerged in Wuhan, China in late 2019. On March 11th, 2020, the World Health Organization (WHO) declared a global COVID-19 pandemic. As of 15th November 2022, SARS-CoV-2 has infected more than 630 million people and claimed the lives of almost 6.6 million patients [1,2,3,4]. COVID-19 presents with a wide range of manifestations ranging from being completely asymptomatic to acute respiratory distress syndrome (ARDS) and eventual death. Among the symptomatic cases, the main presentations include flu-like symptoms such as fever, chills, dry cough, dyspnea, fatigue, myalgia, headache, sore throat, and rhinorrhea [5].
COVID-19 can result in an extensive range of extrapulmonary, and neurological signs and symptoms such as olfactory and/or taste dysfunction (anosmia (loss of smell), ageusia (loss of taste), and dysgeusia (bad taste)), otologic symptoms, dizziness, facial nerve palsy, and long-term neurological complications [6,7,8,9]. In addition, a meta-analysis of 145,721 COVID-19 patients reported 41 different neurologic manifestations, with a pooled prevalence of fatigue, myalgia, taste impairment, smell impairment and headache being 32%, 20%, 21%, 19%, and 13%, respectively [10].
It has been shown that the angiotensin-converting enzyme 2 (ACE2) is the main functional receptor for SARS-CoV-2 and can be found in various body tissues including the gut, blood vessels, heart, lung, brain, testis, and kidneys [11, 12]. In the nervous system, glial cells and neurons commonly express ACE2 which can be a potential target for SARS-CoV-2, and through this receptor the virus can directly or indirectly damage the nervous system [13].Previous studies have implicated viral infections, as a potential cause for both congenital and acquired hearing loss with sensorineural hearing loss being the most prevalent form. However, conductive hearing loss and mixed hearing loss have also been recorded. Viral-caused hearing loss pathogenesis is diverse and has been interpreted as direct inner ear injury due to immune-mediated damage [14]. In addition, since the beginning of the pandemic, many studies have described otologic symptoms including hearing loss, vertigo, and tinnitus among COVID-19 patients, and previous systematic reviews have explored this topic [15, 16]. In addition, in regard to audio-vestibular neurologic symptoms, a meta-analysis of 350 articles by Misra et al. found that the pooled prevalence of hearing impairment and tinnitus among COVID-19 patients was 3% and 5%, respectively [10]. Therefore, in this article, we aimed to conduct an umbrella review on COVID-19 infection and otologic manifestations including sensorineural hearing loss, conductive hearing loss, and mixed hearing loss to broaden our knowledge of their prevalence, treatment, and outcomes among COVID-19 patients.
Methods
For this umbrella review, we focus on currently available systematic review literature investigating hearing loss occurrence in the course of COVID-19 disease. To substantiate the outcomes, this study adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist. Furthermore, methodological quality was assessed by the Amstar-2 quality assessment checklist.
Data sources
The electronic databases used to conduct the online searches using the defined keywords included PubMed, Scopus, Web of Science, and Embase. The database search was conducted on October 15, 2022. With the purpose to identify all pertinent literature the below keywords and their plentiful combinations were used:
-
A.
“COVID-19” OR “SARS-CoV-2” OR “Coronavirus disease 2019” OR “severe acute respiratory syndrome coronavirus 2” [Title/Abstract]
-
B.
“Auditory defect” OR “Hearing damage” OR “Hearing defect” OR “Hearing difficulty” OR “Hearing loss” OR “Impaired hearing” OR “Hypoacusis” OR “Hearing impairment” OR “Transitory deafness” [Title/Abstract]
-
C.
“Systematic review” [Title/ Abstract]
-
D.
[A] AND [B] AND [C]
Study selection
A two-step process was performed for the screening and selection of appropriate articles. First, titles and abstracts of the articles were examined for relevance in our study. Two researchers implemented this step. Second, three researchers diligently evaluated the full texts of potentially eligible publications. The inclusion and exclusion criteria for the articles are below.
Inclusion criteria: English language, systematic review, and peer reviewed prior to acceptance for publication.
Exclusion criteria: Non-human research experiments, duplicated articles, abstracts with inaccessible full texts, conference abstracts, and letters to editors.
Data extraction
Systematic review publications fulfilling the study eligibility criteria were advanced to data extraction. Data of interest consisted of first author’s ID, publication year, searched databases and number of included papers in each publication, methods of auditory system evaluation, total number of COVID-19 patients and patients suffering from simultaneous hearing loss, type of hearing loss, and main findings in each study. This step was executed by three researchers. Throughout the data extraction process, other researchers additionally checked the selected articles/ extracted data, duplicates and possible overlaps.
Quality and risk of bias assessment
To critically appraise the included systematic review studies, we utilized the A MeaSurement Tool to Assess Systematic Reviews 2 (AMSTAR-2). This quality assessment tool applies 16 items to individual studies. These items are visible at the bottom of the table.
Results
Search and selection of studies
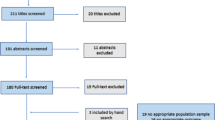
A total of 74 papers were retrieved from the initial search strategy. After the first review of papers, 42 duplicates were identified and removed, and the titles and abstracts of the remaining 32 articles were reviewed by two independent researchers, leading to the extraction of 15 studies for full-text assessment. Next, the remaining studies were evaluated in the full-text screening step by applying the eligibility criteria, and finally, 11 studies met the inclusion criteria and were included in the final review (Fig. 1).
Characteristics of the included studies
Table 1 shows the quality and risk of bias assessment results of the included studies. Among the 11 eligible studies, 8 studies were systematic reviews while the remaining three studies had additional quantitative synthesis and were meta-analyses. The PubMed database was searched by all included studies (n = 11). Moreover, the next most common databases used were: Google Scholar (n = 6), Embase (n = 5), Scopus (n = 5), Web of Science (n = 4), and Cochrane (n = 3). Three included reviews were conducted in Italy, two in India, while the following countries each had one involved paper; UK, Canada, South Africa, Iran, USA, and China. In total, 272 articles were included in these reviews, and the number of enrolled studies of the included reviews ranged between 5 and 102 articles. A total of 116,212 COVID-19 patients were investigated in all 11 studies, and 1,064 cases of hearing loss of all three types were reported. Seven studies specified the hearing loss types, with the most common type being sensorineural hearing loss which was mentioned by six studies (132 cases), followed by conductive hearing loss which was noted by four studies (21 cases), and mixed hearing loss that was enumerated in two articles (three cases). The prevalence rate of hearing loss was reported by three studies which ranged between 0.2% and 7.6% [15, 17, 18]. Yadav et al., in their study of more than 100,000 COVID-19 patients stated that hearing loss was the least prevalent otolaryngological finding [18]. Maharaj et al., in their study of 28 COVID-19-associated hearing loss cases stated that SARS-CoV-2 would result in middle ear infections most probably through the virus spreading into the middle ear and associated neural structures, thus causing sensorineural hearing loss [16].
Audiological evaluation methods
Generally, 13 types of audiological evaluation methods were utilized to evaluate auditory function among subjects, and 10 studies specified their applied tests. These tests were as follows: pure tone audiometry (n = 6), otoacoustic emissions (n = 6), tuning fork tests (n = 2), hearing handicap inventory (n = 2), tinnitus handicap inventory (n = 2), auditory brainstem response (n = 2), dizziness handicap inventory (n = 1), and suppression head impulse paradigm (n = 1).
Treatments and outcomes
A total of 4 treatment strategies were used by different articles as follow: oral corticosteroids, intratympanic corticosteroids, combined oral and intratympanic corticosteroids, and hyperbaric oxygen therapy. Five studies investigated corticosteroid use in the forms of oral or intratympanic injection; four studies reported (complete or partial) hearing improvements after steroid treatment [19,20,21,22], while one study stated no significant improvement in hearing function [15]. One study reported that oral corticosteroid monotherapy alone was not effective, while vestibular symptoms were ameliorated by a combination of oral prednisone, intratympanic dexamethasone injection, and hydroxychloroquine. They also reported that a combination of prednisolone, vitamin B, folic acid, and proton pump inhibitors resulted in complete resolution of hearing loss [21]. Meng et al. concluded that intratympanic injections were safer and probably more effective compared with systemic corticosteroid use [22].
Radiological findings
One study reported the following MRI findings: inner ear damage, bilateral intra-labyrinthine hemorrhage, bilateral cochlear inflammation, and cochlear fibrosis [23]. Details about the included studies and clinical characteristics of COVID-19 patients with hearing loss are shown in Table 2.
Discussion
Since the outbreak of COVID-19, many atypical symptoms have been reported accordingly. Even though the primary site of SARS-CoV-2 is the lung, it can cause various extrapulmonary symptoms, for example, sensory and neural complications such as sudden onset olfactory and gustatory dysfunction, otologic signs (e.g., tinnitus, vertigo, and hearing loss), nonspecific symptoms, and long-term neurological complications [26]. Sudden sensorineural hearing loss with no other symptoms may be one of the earliest complications associated with COVID-19, with an incidence rate of 0.2–7.6%; hence all patients with hearing loss or acute vestibular disease should be screened for SARS-CoV-2 infection [27,28,29]. In this study, we aimed to conduct an umbrella review of current evidence based on the systematic reviews and meta-analyses that examined hearing loss as a complication of COVID-19. Findings from this review may assist decision-makers to have a better understanding of possible pathologies and determination of appropriate medical treatment plan.
Hearing loss classification and diagnosis
There are three types of hearing loss, sensorineural hearing loss, conductive hearing loss, and mixed hearing loss. To differentiate between them, first, we must confirm the genuine existence of hearing loss. The studies in the present review applied various techniques to confirm that the patient had hearing loss. The tests used to verify hearing loss included: pure tone audiometry, otoacoustic emissions, tuning fork tests, hearing handicap inventory, auditory brainstem response, and suppression head impulse paradigm. However, pure tone audiometry was the most frequent test used to diagnose hearing loss. These results are supported by Meng et al., where pure tone audiometry was reported as the most common method for audiological assessment [30].
The most common type of hearing loss was sudden sensorineural hearing loss, which can be defined as the sudden beginning of sensorineural hearing loss without a particular etiology, with at least three consecutive frequency losses ≥ 30 dB within 72 h [30]. Jafari et al. reported unilateral sudden sensorineural hearing loss as the most common type of hearing loss, and only one case report on conductive hearing loss [26]. The occurrence of post-COVID-19 sensorineural hearing loss can be explained by three different mechanisms. Specifically, (1) neuritis caused by the direct invasion of the cochlear nerve by the virus, (2) involvement of cochlea and peri lymphatic tissue that can cause inflammation of cochlea, and (3) the immune reaction caused by the cross reaction between the inner ear and viral antigens [31].
Treatment
It is well known that excessive release of inflammatory cytokines (cytokine storm), especially IL-6 in SARS-COV-2 infection, can cause disease symptoms; therefore, suppression of these reactions is necessary [32]. Based on the results of the present review, the most common type of treatment was corticosteroids due to their suppressive effects on the immune response mentioned above. As an initial treatment, intravenous or intratympanic glucocorticoids can be used for patients separately or in combination. Chandrasekhar et al. investigated the difference between intratympanic and systemic intravenous steroids on sensorineural hearing loss, and they found that intratympanic steroids (often in combination with systemic steroids) had no overall merit to systemic steroids and led to similar results [33]. Nonetheless, this theory might be incorrect, and some findings are debatable. For instance, Tsuda et al., stated that although the two treatments have no significant difference in outcomes, intratympanic steroid therapy has few to almost nothing systemic side effects. Perforation of the tympanic membrane was reported in only one of the 21 patients, suggesting it could be used in outpatient clinics. However, intravenous steroids require hospitalization to prevent causing severe side effects such as insomnia, increased blood pressure, etc. [34]. Other treatment plans include hyperbaric oxygen therapy and cochlear implantation, which can be used when patients do not respond to steroid therapy [35].
Limitations
This study has several limitations. First, the number of studies selected for review in this paper is limited, as the focus was on hearing loss in COVID-19 patients. Second, in some studies, hearing loss may have already existed before the SARS-CoV-2 infection. Lastly, more research is needed with a more extensive study population to provide adequate knowledge.
Conclusions
Despite being one of the rare complications of COVID-19, hearing loss can impact a patient's quality of life. Sensorineural hearing loss is the most common type and it can be diagnosed with various techniques. However, pure tone audiometry is the most frequent technique used. The initial treatment plan may include steroids administered via intravenous or intratympanic. However, some studies preferred intratympanic to intravenous steroids due to the fewer systemic side effects. If patients do not respond to steroids there are various other treatment plans that can be used.
Availability of data and materials
The authors stated that all information provided in this article could be shared.
References
Mehraeen E, Dadras O, Afsahi AM, Karimi A, Pour MM, Mirzapour P et al (2022) Vaccines for COVID-19: a systematic review of feasibility and effectiveness. Infect Disord Drug Targets 22(2):e230921196758
Mehraeen E, Najafi Z, Hayati B, Javaherian M, Rahimi S, Dadras O et al (2022) Current treatments and therapeutic options for COVID-19 patients: a systematic review. Infect Disord Drug Targets 22(1):e260721194968
Mehraeen E, Oliaei S, SeyedAlinaghi S, Karimi A, Mirzapour P, Afsahi AM, et al (2022) COVID-19 in pediatrics: a systematic review of current knowledge and practice. Infect Dis Drug Targ (Formerly Curr Drug Targ Infect Dis) 22(5):47–57
SeyedAlinaghi S, Karimi A, Barzegary A, Pashaei Z, Afsahi AM, Alilou S et al (2022) Mucormycosis infection in patients with COVID-19: a systematic review. Health Sci Rep 5(2):e529
Centers for Disease Control and Prevention (CDC) (2022) Symptoms of COVID-19. Available at: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html
Chen X, Laurent S, Onur OA, Kleineberg NN, Fink GR, Schweitzer F et al (2021) A systematic review of neurological symptoms and complications of COVID-19. J Neurol 268(2):392–402
Abboud H, Abboud FZ, Kharbouch H, Arkha Y, El Abbadi N, El Ouahabi A (2020) COVID-19 and SARS-Cov-2 infection: pathophysiology and clinical effects on the nervous system. World Neurosurg 140:49–53
Cabrera Muras A, Carmona-Abellán M, Collía Fernández A, Uterga Valiente JM, Antón Méndez L, García-Moncó JC (2021) Bilateral facial nerve palsy associated with COVID-19 and epstein-barr virus co-infection. Eur J Neurol 28(1):358–360
Agyeman AA, Chin KL, Landersdorfer CB, Liew D, Ofori-Asenso R (2020) Smell and taste dysfunction in patients with COVID-19: a systematic review and meta-analysis. Mayo Clin Proc 95(8):1621–1631
Misra S, Kolappa K, Prasad M, Radhakrishnan D, Thakur KT, Solomon T et al (2021) Frequency of neurologic manifestations in COVID-19: a systematic review and meta-analysis. Neurology 97(23):e2269–e2281
Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS (2020) Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med 46(4):586–590
Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W (2020) Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. biorxiv.
Mao L, Jin H, Wang M, Hu Y, Chen S, He Q et al (2020) Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA neurology 77(6):683–690
Cohen BE, Durstenfeld A, Roehm PC (2014) Viral causes of hearing loss: a review for hearing health professionals. Trends Hear 18:2331216514541361
Jafari Z, Kolb BE, Mohajerani MH (2022) Hearing loss, tinnitus, and dizziness in COVID-19: a systematic review and meta-analysis. Can J Neurol Sci 49(2):184–195
Maharaj S, Bello Alvarez M, Mungul S, Hari K (2020) Otologic dysfunction in patients with COVID-19: a systematic review. Laryngosc Investig Otolaryngol 5(6):1192–1196
Almufarrij I, Munro KJ (2021) One year on: an updated systematic review of SARS-CoV-2, COVID-19 and audio-vestibular symptoms. Int J Audiol 60(12):935–945
Yadav M, Singh A, Meena J, Sankar JM (2022) A systematic review and meta-analysis of otorhinolaryngologic manifestations of COVID-19 in paediatric patients. J Laryngol Otol:1–61.
De Luca P, Scarpa A, Ralli M, Tassone D, Simone M, De Campora L, et al (2021) Auditory disturbances and SARS-CoV-2 infection: brain inflammation or cochlear affection? Systematic review and discussion of potential pathogenesis. Fronti Neurol:1234.
Umashankar A, Prakash P, Prabhu P (2021) Sudden sensorineural hearing loss post coronavirus disease: a systematic review of case reports. Indian J Otolaryngol Head Neck Surg 1–8.
McIntyre KM, Favre NM, Kuo CC, Carr MM (2021) Systematic review of sensorineural hearing loss associated with COVID-19 infection. Cureus 13(11).
Meng X, Wang J, Sun J, Zhu K (2022) COVID-19 and sudden sensorineural hearing loss: a systematic review. Front Neurol 13.
Fancello V, Hatzopoulos S, Corazzi V, Bianchini C, Skarżyńska MB, Pelucchi S et al (2021) SARS-CoV-2 (COVID-19) and audio-vestibular disorders. Int J Immunopathol Pharmacol 35:20587384211027372
Maleki M, Maarefvand M, Nazeri AR, Baghban ARA, Borna A (2022) Audio-vestibular profile of COVID-19; systematic review and meta-analysis. Iranian J Otorhinolaryngol 34(123):145
De Luca P, Di Stadio A, Colacurcio V, Marra P, Scarpa A, Ricciardiello F et al (2022) Long COVID, audiovestibular symptoms and persistent chemosensory dysfunction: a systematic review of the current evidence. Acta Otorhinolaryngol Ital 42(Suppl 1):S87
Jafari Z, Kolb BE, Mohajerani MH (2022) Hearing loss, tinnitus, and dizziness in COVID-19: a systematic review and meta-analysis. Can J Neurol Sci 49(2):184–195
Kalcioglu MT, Cag Y, Kilic O, Tuysuz O (2020) Can COVID-19 cause sudden sensorineural hearing loss? Int J Infect Dis 101:205
Narożny W, Skorek A, Tretiakow D (2021) Should patients with sudden deafness be tested for COVID19? Auris Nasus Larynx 48(4):797–798
Parrino D, Frosolini A, Toninato D, Matarazzo A, Marioni G, de Filippis C (2022) Sudden hearing loss and vestibular disorders during and before COVID-19 pandemic: an audiology tertiary referral centre experience. Am J Otolaryngol 43(1):103241
Meng X, Wang J, Sun J, Zhu K (2022) COVID-19 and sudden sensorineural hearing loss: a systematic review. Front Neurol 13:883749
Umashankar A, Prakash P, Prabhu P (2022) Sudden sensorineural hearing loss post coronavirus disease: a systematic review of case reports. Indian J Otolaryngol Head Neck Surg 74(Suppl 2):3028–3035
SeyedAlinaghi S, Karimi A, MohsseniPour M, Barzegary A, Mirghaderi SP, Fakhfouri A et al (2021) The clinical outcomes of COVID-19 in HIV-positive patients: a systematic review of current evidence. Immuni Inflamm Dis 9(4):1160–1185
Chandrasekhar SS, Tsai Do BS, Schwartz SR, Bontempo LJ, Faucett EA, Finestone SA et al (2019) Clinical practice guideline: sudden hearing loss (update). Otolaryngol Head Neck Surg. 161(1_suppl):S1-s45
Tsuda T, Hanada Y, Wada K, Fujiwara E, Takeda K, Nishimura H (2021) Efficacy of intratympanic glucocorticoid steroid administration therapy as an initial treatment for idiopathic sudden sensorineural hearing loss during the COVID-19 Pandemic. Ear Nose Throat J:1455613211032534.
De Luca P, Scarpa A, Ralli M, Tassone D, Simone M, De Campora L et al (2021) Auditory disturbances and SARS-CoV-2 infection: brain inflammation or cochlear affection? Systematic review and discussion of potential pathogenesis. Front Neurol 12:707207
Acknowledgements
The present study was conducted in collaboration with Khalkhal University of Medical Sciences, Iranian Research Center for HIV/AIDS, Tehran University of Medical Sciences, and the University of Sydney.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
The conception and design of the study: EM, SASA. Acquisition of data: AA, AMA, RS. Analysis and interpretation of data: AF, KK. Drafting the article: EM, SV, MAH, AM, AD, ZT, KN. Revising it critically for important intellectual content: SASA, DH. Final approval of the version to be submitted: SASA, EM, DH.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this manuscript.
Ethics approval and consent to participate
Not applicable.
Consent to publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mehraeen, E., Afzalian, A., Afsahi, A.M. et al. Hearing loss and COVID-19: an umbrella review. Eur Arch Otorhinolaryngol 280, 3515–3528 (2023). https://doi.org/10.1007/s00405-023-07982-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-023-07982-2