Abstract
Purpose
In this study, we aimed to determine whether or not COM leads to loss of spiral and Scarpa ganglion neurons.
Methods
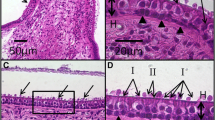
From the human temporal bone (HTB) collection at the University of Minnesota we selected human temporal bones with COM, defined as the presence of clinically intractable tissue abnormalities in the middle ear (cholesteatoma, perforation of the eardrum, granulation tissue, fibrosis, tympanosclerosis, and cholesterol granuloma). We also selected HTBs from donors with no ear diseases as controls. We quantitatively analyzed the number of spiral and Scarpa ganglion cells and compared the results obtained in the control and study groups.
Results
In both COM and control groups we observed a significant negative correlation between age and number of both spiral (R = -0.632; P < 0.001; 95% CI − 0.766 to − 0.434) and Scarpa ganglion (R = − 0.404; P = 0.008; 95% CI − 0.636 to − 0.051) cells. We did not find any significant differences in the number of spiral ganglion cells (in total or per segment) or in the density of Scarpa ganglion cells (in each vestibular nerve or both) in the COM group as compared with controls (P > 0.05).
Conclusions and relevance
Our results did not demonstrate significant loss of cochlear or vestibular peripheral ganglion neuron loss in HTBs with COM as compared with controls.




Similar content being viewed by others
References
Monasta L, Ronfani L, Marchetti F et al (2012) Burden of disease caused by otitis media: systematic review and global estimates. PLoS ONE 7:e36226. https://doi.org/10.1371/journal.pone.0036226
de Penido NO, Chandrasekhar SS, Borin A et al (2016) Complications of otitis media: a potentially lethal problem still present. Braz J Otorhinolaryngol 82:253–262. https://doi.org/10.1016/j.bjorl.2015.04.007
Cordeiro FP, da Costa Monsanto R, Kasemodel ALP et al (2018) Extended high-frequency hearing loss following the first episode of otitis media. Laryngoscope 128:2879–2884. https://doi.org/10.1002/lary.27309
Tucci DL, Wilson BS, O’Donoghue GM (2017) The growing—and now alarming—burden of hearing loss worldwide. Otol Neurotol 38:1387. https://doi.org/10.1097/MAO.0000000000001593
Aarhus L, Tambs K, Hoffman HJ, Engdahl B (2016) Childhood otitis media is associated with dizziness in adulthood: the HUNT cohort study. Eur Arch Otorhinolaryngol 273:2047–2054. https://doi.org/10.1007/s00405-015-3764-9
Aarhus L, Homøe P, Engdahl B (2020) Otitis media in childhood and disease in adulthood: a 40-year follow-up study. Ear Hear 41:67–71. https://doi.org/10.1097/AUD.0000000000000729
Paparella MM, Oda M, Hiraide F, Brady D (1972) Pathology of sensorineural hearing loss in otitis media. Ann Otol Rhinol Laryngol 81:632–647. https://doi.org/10.1177/000348947208100503
da Costa SS, Paparella MM, Cruz OLM, Rollin GAFS (1995) Chronic otitis media: a clinical-histopathological correlation. Otolaryngol Neck Surg 113:P110–P110. https://doi.org/10.1016/S0194-5998(05)80735-1
Livingston G, Sommerlad A, Orgeta V et al (2017) Dementia prevention, intervention, and care. Lancet 390:2673–2734. https://doi.org/10.1016/S0140-6736(17)31363-6
Lin FR, Metter EJ, O’Brien RJ et al (2011) Hearing loss and incident dementia. Arch Neurol 68:214–220. https://doi.org/10.1001/archneurol.2010.362
Paparella MM (1991) Interactive inner-ear/middle-ear disease, including perilymphatic fistula. Acta Otolaryngol Suppl 485:36–45
Cureoglu S, Schachern PA, Paparella MM, Lindgren BR (2004) Cochlear changes in chronic otitis media. Laryngoscope 114:622–626. https://doi.org/10.1097/00005537-200404000-00006
Goycoolea MV (2001) Clinical aspects of round window membrane permeability under normal and pathological conditions. Acta Otolaryngol (Stockh) 121:437–447
Goycoolea MV, Paparella MM, Goldberg B et al (1980) Permeability of the middle ear to staphylococcal pyrogenic exotoxin in otitis media. Int J Pediatr Otorhinolaryngol 1:301–308
Goycoolea MV, Muchow D, Schachern P (1988) Experimental studies on round window structure: function and permeability. Laryngoscope 98:1–20. https://doi.org/10.1288/00005537-198806001-00002
Monsanto RC, Schachern P, Paparella MM et al (2017) Progression of changes in the sensorial elements of the cochlear and peripheral vestibular systems: the otitis media continuum. Hear Res 351:2–10. https://doi.org/10.1016/j.heares.2017.05.003
da Costa Monsanto R, Erdil M, Pauna HF et al (2016) Pathologic changes of the peripheral vestibular system secondary to chronic otitis media. Otolaryngol Head Neck Surg 155:494–500. https://doi.org/10.1177/0194599816646359
Kodama A, Ishii T, Oka Y, Uebo K (1988) Histopathology of the inner ear in chronic otitis media. Equilibr Res 47:94–100
Kaya S, Tsuprun V, Hizli Ö et al (2016) Quantitative assessment of cochlear histopathologic findings in patients with suppurative labyrinthitis. JAMA Otolaryngol Head Neck Surg 142:364–369. https://doi.org/10.1001/jamaoto.2015.3803
Schachern PA, Paparella MM, Hybertson R et al (1992) Bacterial tympanogenic labyrinthitis, meningitis, and sensorineural damage. Arch Otolaryngol Head Neck Surg 118:53–57
Paparella MM, Schachern PA, Yoon TH et al (1990) Otopathologic correlates of the continuum of otitis media. Ann Otol Rhinol Laryngol Suppl 148:17–22
Otte J, Schunknecht HF, Kerr AG (1978) Ganglion cell populations in normal and pathological human cochleae. Implications for cochlear implantation. Laryngoscope 88:1231–1246. https://doi.org/10.1288/00005537-197808000-00004
Richter E (1980) Quantitative study of human Scarpa’s ganglion and vestibular sensory epithelia. Acta Otolaryngol (Stockh) 90:199–208
Abercrombie M (1946) Estimation of nuclear population from microtome sections. Anat Rec 94:239–247
Casselbrant ML, Furman JM, Rubenstein E, Mandel EM (1995) Effect of otitis media on the vestibular system in children. Ann Otol Rhinol Laryngol 104:620–624. https://doi.org/10.1177/000348949510400806
Cureoglu S, Schachern PA, Rinaldo A et al (2005) Round window membrane and labyrinthine pathological changes: an overview. Acta Otolaryngol (Stockh) 125:9–15. https://doi.org/10.1080/00016480410022534
Goycoolea MV, Lundman L (1997) Round window membrane. Structure function and permeability: a review. Microsc Res Tech 36:201–211. https://doi.org/10.1002/(SICI)1097-0029(19970201)36:3%3c201:AID-JEMT8%3e3.0.CO;2-R
MacArthur CJ, Pillers D-AM, Pang J et al (2011) Altered expression of middle and inner ear cytokines in mouse otitis media. Laryngoscope 121:365–371. https://doi.org/10.1002/lary.21349
MacArthur CJ, Hausman F, Kempton JB et al (2013) Otitis Media Impacts Hundreds of Mouse Middle and Inner Ear Genes. PLoS ONE 8:e75213. https://doi.org/10.1371/journal.pone.0075213
Paparella MM, Goycoolea MV, Meyerhoff WL, Shea D (1979) Endolymphatic hydrops and otitis media. Laryngoscope 89:43–58
Makary CA, Shin J, Kujawa SG et al (2011) Age-related primary cochlear neuronal degeneration in human temporal bones. J Assoc Res Otolaryngol 12:711–717. https://doi.org/10.1007/s10162-011-0283-2
Linthicum FH, Fayad J (2009) Spiral ganglion cell loss is unrelated to segmental cochlear sensory system degeneration in humans. Otol Neurotol 30:418–422
Kaya S, Tsuprun V, Hızlı Ö et al (2016) Cochlear changes in serous labyrinthitis associated with silent otitis media: a human temporal bone study. Am J Otolaryngol 37:83–88. https://doi.org/10.1016/j.amjoto.2015.10.002
Suzuka Y, Schuknecht HF (1988) Retrograde cochlear neuronal degeneration in human subjects. Acta Otolaryngol Suppl 450:1–20
Zilberstein Y, Liberman MC, Corfas G (2012) Inner hair cells are not required for survival of spiral ganglion neurons in the adult cochlea. J Neurosci 32:405–410. https://doi.org/10.1523/JNEUROSCI.4678-11.2012
Liberman MC, Liberman LD, Maison SF (2015) Chronic conductive hearing loss leads to cochlear degeneration. PLoS ONE 10:e0142341. https://doi.org/10.1371/journal.pone.0142341
Okada M, Welling DB, Liberman MC, Maison SF (2019) Chronic conductive hearing loss is associated with speech intelligibility deficits in patients with normal bone conduction thresholds. Ear Hear. https://doi.org/10.1097/AUD.0000000000000787
Paparella MM, Shea D, Meyerhoff WL, Goycoolea MV (1980) Silent otitis media. Laryngoscope 90:1089–1098. https://doi.org/10.1288/00005537-198007000-00003
da Monsanto RC, Kasemodel ALP, Tomaz A et al (2018) Current evidence of peripheral vestibular symptoms secondary to otitis media. Ann Med 50:391–401. https://doi.org/10.1080/07853890.2018.1470665
Haapala S, Niemitalo-Haapola E, Raappana A et al (2014) Effects of recurrent acute otitis media on cortical speech-sound processing in 2-year old children. Ear Hear 35:e75–e83. https://doi.org/10.1097/AUD.0000000000000002
Macandie C, O’Reilly BF (1999) Sensorineural hearing loss in chronic otitis media. Clin Otolaryngol 24:220–222. https://doi.org/10.1046/j.1365-2273.1999.00237.x
Liberman MC, Kujawa SG (2017) Cochlear synaptopathy in acquired sensorineural hearing loss: manifestations and mechanisms. Hear Res 349:138–147. https://doi.org/10.1016/j.heares.2017.01.003
Kujawa SG, Liberman MC (2015) Synaptopathy in the noise-exposed and aging cochlea: primary neural degeneration in acquired sensorineural hearing loss. Hear Res 330:191–199. https://doi.org/10.1016/j.heares.2015.02.009
Guitton MJ (2012) Tinnitus: pathology of synaptic plasticity at the cellular and system levels. Front Syst Neurosci. https://doi.org/10.3389/fnsys.2012.00012
Durán Alonso MB, Feijoo-Redondo A, Conde de Felipe M et al (2012) Generation of inner ear sensory cells from bone marrow-derived human mesenchymal stem cells. Regen Med 7:769–783. https://doi.org/10.2217/rme.12.65
Acknowledgements
We thank our supporters: the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES—Brazil) (Finance code: 001), the National Institute of Neurological Disorders and Stroke of the NIH (Grant No. UG3NS107688), the International Hearing Foundation, the 5 M Lions International, and the Starkey Foundation.
Funding
RCM received a scholarship from the “Coordenação de aperfeiçoamento pessoal de nível superior” (CAPES) (Finance code: 001). The research reported in this manuscript was supported by the National Institute of Neurological Disorders and Stroke of the NIH (UG3NS107688), the International Hearing Foundation, the 5 M Lions International, and the Starkey Foundation.
Author information
Authors and Affiliations
Contributions
All authors performed similar contributions and were involved in all steps of the manuscript production. RCM, NOP and MU performed the literature review, analyzed the human temporal bones and wrote the final version of the manuscript. PS, MMP, and SC were the intellectual authors of the work, supervised the collection of data, analyzed the results and critically reviewed the final version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
All authors declare that they have no conflict of interest.
Ethical approval
The Institutional Review Board of the University of Minnesota (0206M26181) and Universidade Federal de São Paulo/Escola Paulista de Medicina (UNIFESP/EPM) (1.751.916) approved this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Monsanto, R.C., Penido, N.O., Uchiyama, M. et al. Quantitative assessment of cochlear and vestibular ganglion neurons in temporal bones with chronic otitis media. Eur Arch Otorhinolaryngol 278, 331–338 (2021). https://doi.org/10.1007/s00405-020-06094-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-020-06094-5