Abstract
Purpose
Early facial nerve palsy (eFNP) is the most frequent complication of the parotidectomy. Intraoperative neuromonitoring (IONM) in parotid surgery, which aims at reducing eFNP, has not evolved any further than the mere differentiation between the nerve and the surrounding tissue. Continuous IONM (cIONM), used in thyroid and posterior fossa surgery, has developed over the past years and has proved beneficial in reducing the rate of paresis in cases where a pattern of impending nerve injury is identified. In this study, we aim to demonstrate the safety of using the stimulating electrode (Saxophone®) for cIONM in parotid surgery.
Methods
From 2016 to 2018, 40 patients who were referred for primary parotidectomy under cIONM according to our study protocol (registered at the German Clinical Trials Register, DRKS-ID: DRKS00011051, http://www.drks.de; http://apps.who.int/trialsearch) were included in this study. All patients with a normal preoperative facial nerve function [House–Brackman (HB)-Index 1] underwent surgery using continuous facial nerve stimulation with the Saxophone® electrode (system AVALANCHE XT, Dr. Langer Medical, Waldkirch, Germany). A control group which underwent parotidectomies with only intermittent IONM was recruited from our records.
Results
Half of the patients in our study group suffered from eFNP. All except one regained normal facial nerve function within 6 months of surgery. There was no significant difference regarding eFNP when compared to the control group without cIONM (p = 0.11). No statistically significant correlation between the stimulation threshold (p = 0.74) or the duration of nerve stimulation and eFNP was found (p = 0.51).
Conclusion
We have demonstrated the safety of using the Saxophone®-electrode for cIONM of the facial nerve in parotid surgery. Future development of this method could enable the recognition of impending nerve injury and thus reduce eFNP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Early facial nerve palsy (eFNP) is the most frequent complication of parotidectomy with reported rates of 28.8–77.2% in primary surgery [1,2,3,4,5]. This pertains to superficial lateral or partial superficial lateral parotidectomy with nerve dissection. It has been shown that eFNP has a significant impact on the physical and psychosocial well-being of the patient [5].
In many institutions, efforts have been made to reduce the rate of eFNP by making use of intraoperative neuromonitoring (IONM). A recent meta-analysis showed IONM to significantly reduce eFNP [6]. Although the history of IONM started in 1969 [7], no significant breakthrough in the method has been noted and consequently IONM in parotid surgery is used merely to differentiate between the nerve and the surrounding tissue.
Novel methods of continuous IONM (cIONM) in which the vagus and the facial nerve are continuously stimulated to acquire “real-time” information of the nerve function have evolved in other surgical procedures, though mainly in thyroidectomy and surgery on the posterior cranial fossa. Patterns of impending nerve injury are thereby identifiable, providing a signal to the surgeon who is then able to modify the causative surgical movement and thus avoid nerve injury [8, 9].
Inspired by these findings, we have aimed to implement this method in parotid surgery, seek out a pattern of nerve injury and integrate this pattern into an algorithm which will then warn the surgeon of impending nerve injury.
Material and methods
A prospective, non-randomized study was carried out and the results thereof compared to a retrospective cohort from our department. All patients enrolled in the study provided informed written consent. Ethical approval of the study protocol was obtained from the Saxonian Chamber of Physicians, Germany (25 August 2016, EK-BR-53/16-1). The study was registered at the German Clinical Trials Register prior to conducting the research (ID: DRKS00011051, http://www.drks.de; http://apps.who.int/trialsearch). From October 2016 to October 2018, all parotid surgery patients admitted to our tertiary care hospital were checked for their potential eligibility to enroll in this study.
Inclusion criteria were: preoperative intact function of the facial nerve on both sides [according to House–Brackmann (HB)-Grade 1 [10]], no previous parotid surgery, signed informed consent, and an age of over 18 years. The exclusion criteria were preoperative facial paresis (HB ≥ 2), revision surgery, an age of under 18 years, total parotidectomy with resection of the facial nerve, pace-maker or defibrillator, pregnancy, epilepsy, and heart rhythm problems. The evaluation of the facial nerve function was obtained preoperatively from two independent clinicians and photo documentation.
All surgery was performed under general anesthesia. Prior to skin incision, a pair of monopolar needles were placed in the facial musculature in order to provide four channel EMG-neuromonitoring (frontalis, orbicularis oculi, nasalis, and orbicularis oris muscle; neuromonitoring System: Avalanche® XT [Software Avalanche® SI], Dr. Langer Medical, Waldkirch/Germany).
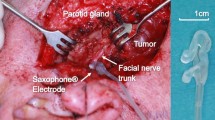
The parotidectomies were performed in the usual anterograde dissection manner after performing the Blair skin incision. Three consultant surgeons operated on all patients using binocular loupes as an optical aid. After identifying the facial nerve trunk, the entire trunk was exposed in every direction by careful preparation with an atraumatic instrument. The Saxophone® electrode (Fig. 1a, Dr. Langer Medical, Waldkirch/Germany) was then placed on the facial nerve trunk (Fig. 1b). The current threshold was then gradually calibrated in 0.1 mA increments, beginning at 0.1 mA, but never exceeding 1 mA, until the amplitudes of the four channels reached maximum values. The nerve trunk was then continuously stimulated by the calibrated current during the preparation of the nerve and the removal of the tumour at a frequency of 3 Hz and a pulse width of 200 µs. The moment the tumour was removed, the Saxophone® electrode was detached. All information obtained during the operation, that is the amplitudes and latencies of the four channels, was automatically recorded in the neuromonitoring device. The facial nerve function was evaluated on the first postoperative day as well as 1 month and six months postoperatively by three independent observers.
Statistical analysis was performed using MedCalc for Windows, version 18.11.3 (MedCalc Software, Ostend/Belgium). To compare the early paresis rate in our prospective study to patients who had undergone surgery without the Saxophone® electrode, we retrospectively analyzed the last 40 patients prior to the study who underwent a parotidectomy with intermittent IONM (iIONM,) using an electrode, applying the same inclusion and exclusion criteria. Fisher’s exact test was applied to calculate statistical significance. Correlation was calculated according to Pearson’s correlation coefficient r and presented with a 95% confidence interval. A p value of < 0.05 was set for statistical significance in all of the above-mentioned methods.
Results
Between October 2016 and October 2018, 51 parotidectomies were performed. 11 patients were excluded from the study. The reasons for exclusion were as follows: implanted defibrillator, rejected taking part in the study and signing informed consent, intraoperative sacrifice of the facial nerve due to malignant involvement (each excluding two patients); fibrosis of the facial nerve trunk, pseudocholinesterase deficiency, revision operation, preoperative facial nerve palsy, and no identification of the facial nerve trunk (each excluding one patient). The facial nerve was stimulated supramaximal with a threshold of 0.62 ± 0.17 mA for 37.4 ± 16.7 min. The placement time of the Saxophone® electrode was 257 ± 152.3 s while the electrode was displaced 1.4 ± 1.9 times. The postoperative histology showed a benign tumour in 37 (92.5%) cases. The average tumour volume was 9.7 ± 15.7 ml (Table 1).
The facial nerve function on the first postoperative day was as follows: 20 patients (50%) with HB 1, 18 patients (45%) with HB 2 and 2 patients (5%) with HB 3. No patients exhibited decreased facial nerve function (HB 4–5). One month postoperatively, 29 patients displayed an HB-grade 1 (72.5%), 10 patients an HB 2 (25%) and 1 patient an HB 3 (2.5%). Six months postoperatively, all but 1 patient improved to HB-grade 1 (39 patients HB 1–97.5%; 1 patient HB 3–2.5%; Fig. 2).
When one compared the last 40 parotidectomies performed prior to this prospective study without the Saxophone® electrode (iIONM) to this cohort, using the same inclusion and exclusion criteria, no statistically significant difference was found with regard to facial nerve function (Table 2, p = 0.11) on the first postoperative day.
No statistically significant correlation between the stimulation threshold (r = 0.05, p = 0.74, 95% CI − 0.26 to 0.36, Fig. 3) or the duration of nerve stimulation and early facial paresis was found (r = − 0.12, p = 0.51, 95% CI − 0.41 to 0.21, Fig. 4).
Discussion
Intraoperative neuromonitoring is a well-established technique in several surgical procedures which contributes to the preservation of neural structures and their functioning. Recent research has developed IONM using continuous monitoring of the nerve function, changing it from a nerve identification tool to a functional monitoring method. Thereby, hazards to the nerve, caused by stretching for example, can be detected and the surgical manipulation adapted to preserve the nerve function. This has mainly been established in thyroid surgery and in surgical procedures involving the neural structures of the cerebello-pontine angle in the posterior cranial fossa.
We have adopted this approach to present a novel method of continuous facial nerve trunk stimulation in parotidectomy. In our study, we have aimed to demonstrate the safety of this cIONM by comparing the results of a prospective study cohort to a retrospective patient group who had solely undergone intermittent IONM. cIONM of the facial nerve of up to 1 mA proved a safe procedure and was not connected to a higher rate of eFNP. Furthermore, neither the threshold of stimulation nor the length of stimulation correlated to decreased postoperative facial nerve function. The cIONM was carried out with a threshold of 0.62 ± 0.17 mA, which enabled supramaximal stimulation, and which was lower than the usual 1 mA threshold used during iIONM.
Bearing in mind that the amount of acquired information in amplitudes and latencies in four channel monitoring at 3 Hz for the average duration of cIONM (37.4 min) comes to over 50,000 per operation, it is clear that computerized data processing is essential for further analysis. However, scrolling through the amplitude values, it is apparent that patients with normal facial nerve function postoperatively have a stable amplitude, whereas patients with eFNP show a drop of amplitude (DOA) of > 50% (Fig. 5). No loss of signal (LOS) was noted in our study group.
Simultaneous depiction of the amplitude of m. orbicularis oris during the entire parotidectomy: a stable amplitude in a patient with postoperative facial nerve function grade 1 according to House–Brackmann; b drop of amplitude of > 50% (red arrow) in a patient with postoperative facial nerve function grade 2 according to House–Brackmann
Once a cut-off value for DOA and a rise in latency that correlates of eFNP is found, this could potentially be integrated into the software of the IONM device to warn the surgeon of impending nerve injury, following which the surgeon could modify the causative surgical maneuver or, for example, administer intravenous corticoids.
The postoperative facial nerve function was normal (HB1) in 50% of patients, while 45% of patients suffered a mild facial palsy (HB2) and 5% of patients had an HB3. Only one patient, who suffered from a malignant tumour, remained HB3 for 6 months postoperatively whereas all others improved to HB1. The reported rate of an eFNP of 50% was in accordance with relevant literature [1,2,3,4,5]. There was also no statistically significant difference when comparing the eFNP in this prospective cohort to the last 40 parotidectomies performed in our institution before the prospective study, using the same inclusion and exclusion criteria (p = 0.11). Although the eFNP rate in the cIONM group was higher, one should bear in mind that these patients were part of a prospective study, whereas the data for the iIONM group was collected retrospectively. It is a common finding in relevant literature that retrospective studies [11,12,13] tend to report lower eFNP rates when compared to prospective studies [1, 4, 5]. This inconsistent reporting of eFNP intensifies the need for objective reporting, such as computerized facial movement recognition [14, 15].
It is dissatisfactory for any surgeon conducting parotid surgery to be confronted with a patient suffering eFNP when no direct damage to the nerve occurred during dissection. That being so, it is therefore clear that intraoperative traction and stretching of the nerve play a crucial role in postoperative nerve function. This has led us to conclude that one can acquire important information on the status of the nerve by continuously stimulating the nerve and analyzing the relevant amplitudes and latencies. In our opinion, the said information will lead to a reduction in eFNP.
The application of cIONM in thyroid surgery has enabled the identification of impending nerve injury and has had a prognostic value in the postoperative palsy of the recurrent nerve [8, 9]. A DOA of > 50% together with a rise of latency of > 10% as well as complete LOS have been identified as signaling impending nerve injury. This pattern has served as a warning to surgeons in subsequent studies and has been used to modify the causative surgical maneuver in 73–82% of cases, thus reducing the postoperative palsy rate significantly [9, 16]. In a high-volume study comparing cIONM to iIONM, significantly more permanent vocal cord palsy was seen in the iIONM group [16].
Furthermore, various methods of cIONM of the facial nerve can be found in literature. These include continuous stimulation of the nerve at the root exit zone during posterior fossa surgery where a negative predictive value of a drop of amplitude of > 50% was noted [17], and transcranial multi-pulse electric stimulation of the corticobulbar pathway to stimulate the facial nerve, used by other authors in the same type of surgery. Here, cut-off values for a drop of amplitude of 50%, 35% and 0% correlated with eFNP [18]. Finally, methods of percutaneous stimulation of the facial nerve during surgery for facial vascular malformations have shown that a DOA of > 50% should serve as an alarm signal to the surgeon to stop the manipulation and to wait until the amplitude normalizes [19].
Limitations
Due to the design of the electrode used in our study, a 360° facial trunk exposure was needed. Although the dissection of the facial nerve trunk was done meticulously using loupe lenses, in some patients this was very difficult due to the anatomy or the fact that the tumour was located directly laterally to the nerve trunk. In these cases, it is possible that unnecessary trauma was inflicted on the nerve due to the effort of implanting the electrode.
Difficulties in deriving the signals were encountered when bleeding in the wound bed or accidental electrode dislocation (1.4 per parotidectomy) occurred. In some patients, due to anatomical circumstances, electrode implantation was difficult. An average of 4.3 min was necessary for the placement. A new electrode design which enables adhesive implantation without the need to dissect the medial aspect of the nerve trunk and which is tailored to the facial nerve is needed.
In patients where fibrosis of the facial nerve trunk was present, implantation of the Saxophone® electrode was not possible, as seen in one patient in our cohort.
Conclusion
We have demonstrated the safe use of a novel method of cIONM of the facial nerve in parotid surgery. cIONM with the Saxophone® electrode is a safe and easy method where neither length nor threshold of stimulation correlates to eFNP. Future development of this method could enable the recognition of impending nerve injury thus reduce eFNP.
Change history
06 March 2020
In the original publication of the article, first name and last names of all authors were swapped.
References
Grosheva M, Klussmann JP, Grimminger C, Wittekindt C, Beutner D, Pantel M, Volk GF, Guntinas-Lichius O (2009) Electromyographic facial nerve monitoring during parotidectomy for benign lesions does not improve the outcome of postoperative facial nerve function: a prospective two-center trial. Laryngoscope 119(12):2299–2305. https://doi.org/10.1002/lary.20637
Graciano AJ, Fischer CA, Coelho GV, Steck JH, Paschoal JR, Chone CT (2018) Facial nerve dysfunction after superficial parotidectomy with or without continuous intraoperative electromyographic neuromonitoring: a prospective randomized pilot study. Eur Arch Otorhinolaryngol 275(11):2861–2868. https://doi.org/10.1007/s00405-018-5130-1
Savvas E, Hillmann S, Weiss D, Koopmann M, Rudack C, Alberty J (2016) Association between facial nerve monitoring with postoperative facial paralysis in parotidectomy. JAMA Otolaryngol Head Neck Surg 142(9):828–833. https://doi.org/10.1001/jamaoto.2016.1192
Ruohoalho J, Makitie AA, Aro K, Atula T, Haapaniemi A, Keski-Santti H, Takala A, Back LJ (2017) Complications after surgery for benign parotid gland neoplasms: a prospective cohort study. Head Neck 39(1):170–176. https://doi.org/10.1002/hed.24496
Prats-Golczer VE, Gonzalez-Cardero E, Exposito-Tirado JA, Montes-Latorre E, Gonzalez-Perez LM, Infante-Cossio P (2017) Impact of dysfunction of the facial nerve after superficial parotidectomy: a prospective study. Br J Oral Maxillofac Surg 55(8):798–802. https://doi.org/10.1016/j.bjoms.2017.07.006
Sood AJ, Houlton JJ, Nguyen SA, Gillespie MB (2015) Facial nerve monitoring during parotidectomy: a systematic review and meta-analysis. Otolaryngol Head Neck Surg 152(4):631–637. https://doi.org/10.1177/0194599814568779
Flisberg K, Lindholm T (1969) Electrical stimulation of the human recurrent laryngeal nerve during thyroid operation. Acta Otolaryngol Suppl 263:63–67
Schneider R, Randolph GW, Sekulla C, Phelan E, Thanh PN, Bucher M, Machens A, Dralle H, Lorenz K (2013) Continuous intraoperative vagus nerve stimulation for identification of imminent recurrent laryngeal nerve injury. Head Neck 35(11):1591–1598. https://doi.org/10.1002/hed.23187
Phelan E, Schneider R, Lorenz K, Dralle H, Kamani D, Potenza A, Sritharan N, Shin J, Randolph W (2014) Continuous vagal IONM prevents recurrent laryngeal nerve paralysis by revealing initial EMG changes of impending neuropraxic injury: a prospective, multicenter study. Laryngoscope 124(6):1498–1505. https://doi.org/10.1002/lary.24550
House JW, Brackmann DE (1985) Facial nerve grading system. Otolaryngol Head Neck Surg 93(2):146–147. https://doi.org/10.1177/019459988509300202
Deneuve S, Quesnel S, Depondt J, Albert S, Panajotopoulos A, Gehanno P, Barry B (2010) Management of parotid gland surgery in a university teaching hospital. Eur Arch Otorhinolaryngol 267(4):601–605. https://doi.org/10.1007/s00405-009-1088-3
Witt R (1998) Facial nerve monitoring in parotid surgery: the standard of care? Otolaryngol Head Neck Surg 119:468–470
Guntinas-Lichius O, Klussmann JP, Wittekindt C, Stennert E (2006) Parotidectomy for benign parotid disease at a university teaching hospital: outcome of 963 operations. Laryngoscope 116(4):534–540. https://doi.org/10.1097/01.mlg.0000200741.37460.ea
Volk GF, Schaede RA, Thielker J, Modersohn L, Mothes O, Nduka CC, Barth JM, Denzler J, Guntinas-Lichius O (2018) Reliability of grading of facial palsy using a video tutorial with synchronous video recording. Laryngoscope. https://doi.org/10.1002/lary.27739
Banks CA, Jowett N, Hadlock TA (2017) Test–retest reliability and agreement between in-person and video assessment of facial mimetic function using the eFACE facial grading system. JAMA Facial Plast Surg 19(3):206–211. https://doi.org/10.1001/jamafacial.2016.1620
Schneider R, Sekulla C, Machens A, Lorenz K, Nguyen Thanh P, Dralle H (2015) Postoperative vocal fold palsy in patients undergoing thyroid surgery with continuous or intermittent nerve monitoring. Br J Surg 102(11):1380–1387. https://doi.org/10.1002/bjs.9889
Amano M, Kohno M, Nagata O, Taniguchi M, Sora S, Sato H (2011) Intraoperative continuous monitoring of evoked facial nerve electromyograms in acoustic neuroma surgery. Acta Neurochir (Wien) 153(5):1059–1067. https://doi.org/10.1007/s00701-010-0937-6(discussion 1067)
Dong C, MacDonald D, Akagami R, Westerberg B, AlKhani A, Kanaan I, Hassounah M (2005) Intraoperative facial motor evoked potential monitoring with transcranial electrical stimulation during skull base surgery. Clin Neurophysiol 116:588–596. https://doi.org/10.1016/j.clinph.2004.09.013
Ulkatan S, Waner M, Arranz-Arranz B, Weiss I, Theresa O, Saral M, Deletis V, Berenstein A (2014) New methodology for facial nerve monitoring in extracranial surgeries of vascular malformations. Clin Neurophysiol 125(4):849–855. https://doi.org/10.1016/j.clinph.2013.08.030
Acknowledgements
Open Access funding provided by Projekt DEAL.
Funding
This project was funded entirely by the Department of Otorhinolaryngology at the Sana Kliniken Leipziger Land, Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have indicated that they have no conflict of interest.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study passed an evaluation of the Ethical Committee of Saxonian Chamber of Physicians. Data handling in accordance with national data protection laws and the anonymity of the patients was secured.
Informed consent
An extended informed consent regarding the study and data analysis was obtained before inclusion to the study, for the retrospective chart analysis an informed consent was obtained according to a treatment contract from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised. The first name and last name of all authors were interchanged and corrected in this version.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stankovic, P., Wittlinger, J., Georgiew, R. et al. Safety of the “Saxophone®” electrode in parotid surgery for continuous intraoperative neuromonitoring of the facial nerve: results of a pro- and retrospective cohort study. Eur Arch Otorhinolaryngol 277, 1449–1456 (2020). https://doi.org/10.1007/s00405-020-05803-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-020-05803-4