Abstract
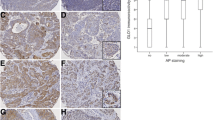
Glutaminolysis is a crucial factor for tumor metabolism in the carcinogenesis of several tumors but has not been clarified for oral squamous cell carcinoma (OSCC) yet. Expression of glutaminolysis-related solute carrier family 1, member 5 (SLC1A5)/neutral amino acid transporter (ASCT2), glutaminase (GLS), and glutamate dehydrogenase (GLDH) was analyzed in normal oral mucosa (n = 5), oral precursor lesions (simple hyperplasia, n = 11; squamous intraepithelial neoplasia, SIN I–III, n = 35), and OSCC specimen (n = 42) by immunohistochemistry. SLC1A5/ASCT2 and GLS were significantly overexpressed in the carcinogenesis of OSCC compared with normal tissue, while GLDH was weakly detected. Compared with SIN I–III SLC1A5/ASCT2 and GLS expression were significantly increased in OSCC. GLDH expression did not significantly differ from SIN I–III compared with OSCC. This study shows the first evidence of glutaminolysis-related SLC1A5/ASCT2, GLS, and GLDH expression in OSCC. The very weak GLDH expression indicates that glutamine metabolism is rather related to nucleotide or protein/hexosamine biosynthesis or to the function as an antioxidant (glutathione) than to energy production or generation of lactate through entering the tricarboxylic acid cycle. Overcoming glutaminolysis by targeting c-Myc oncogene (e.g. by natural compounds) and thereby cross-activation of mammalian target of rapamycin complex 1 or SLC1A5/ASCT2, GLS inhibitors may be a useful strategy to sensitize cancer cells to common OSCC cancer therapies.






Similar content being viewed by others
Abbreviations
- SIN:
-
Squamous intraepithelial neoplasia
- OSCC:
-
Oral squamous cell carcinoma
- SLC1A5:
-
Solute carrier family 1, member 5
- GLS:
-
Glutaminase
- GLDH:
-
Glutamate dehydrogenase
- TCA:
-
Tricarboxylic acid cycle
- mTOR:
-
Mammalian target of rapamycin
References
Grimm M, Cetindis M, Lehmann M, Biegner T, Munz A, Teriete P, Kraut W, Reinert S (2014) Association of cancer metabolism-related proteins with oral carcinogenesis-indications for chemoprevention and metabolic sensitizing of oral squamous cell carcinoma? J Transl Med 12:208. doi:10.1186/1479-5876-12-208
Hensley CT, Wasti AT, DeBerardinis RJ (2013) Glutamine and cancer: cell biology, physiology, and clinical opportunities. J Clin Investig 123(9):3678–3684. doi:10.1172/JCI69600
Alfarouk KO, Shayoub ME, Muddathir AK, Elhassan GO, Bashir AH (2011) Evolution of tumor metabolism might reflect carcinogenesis as a reverse evolution process (dismantling of multicellularity). Cancers (Basel) 3(3):3002–3017. doi:10.3390/cancers3033002
Ogawa T, Washio J, Takahashi T, Echigo S, Takahashi N (2014) Glucose and glutamine metabolism in oral squamous cell carcinoma: insight from a quantitative metabolomic approach. Oral Surg Oral Med Oral Pathol Oral Radiol 118(2):218–225. doi:10.1016/j.oooo.2014.04.003
Sandulache VC, Ow TJ, Pickering CR, Frederick MJ, Zhou G, Fokt I, Davis-Malesevich M, Priebe W, Myers JN (2011) Glucose, not glutamine, is the dominant energy source required for proliferation and survival of head and neck squamous carcinoma cells. Cancer 117(13):2926–2938. doi:10.1002/cncr.25868
Romero-Garcia S, Lopez-Gonzalez JS, Baez-Viveros JL, Aguilar-Cazares D, Prado-Garcia H (2011) Tumor cell metabolism: an integral view. Cancer Biol Ther 12(11):939–948. doi:10.4161/cbt.12.11.18140
Mates JM, Segura JA, Campos-Sandoval JA, Lobo C, Alonso L, Alonso FJ, Marquez J (2009) Glutamine homeostasis and mitochondrial dynamics. Int J Biochem Cell Biol 41(10):2051–2061. doi:10.1016/j.biocel.2009.03.003
Newsholme P, Procopio J, Lima MM, Pithon-Curi TC, Curi R (2003) Glutamine and glutamate—their central role in cell metabolism and function. Cell Biochem Funct 21(1):1–9. doi:10.1002/cbf.1003
Goto M, Miwa H, Shikami M, Tsunekawa-Imai N, Suganuma K, Mizuno S, Takahashi M, Mizutani M, Hanamura I, Nitta M (2014) Importance of glutamine metabolism in leukemia cells by energy production through TCA cycle and by redox homeostasis. Cancer Investig 32(6):241–247. doi:10.3109/07357907.2014.907419
Deberardinis RJ, Sayed N, Ditsworth D, Thompson CB (2008) Brick by brick: metabolism and tumor cell growth. Curr Opin Genet Dev 18(1):54–61. doi:10.1016/j.gde.2008.02.003
Driemel O, Hertel K, Reichert TE, Kosmehl H (2006) Current classification of precursor lesions of oral squamous cell carcinoma principles of the WHO classification 2005. Mund Kiefer Gesichtschir 10(2):89–93. doi:10.1007/s10006-006-0675-3
Tanaka T, Tanaka M, Tanaka T (2011) Oral carcinogenesis and oral cancer chemoprevention: a review. Pathol Res Int 2011:431246. doi:10.4061/2011/431246
Sobin LH, Wittekind Ch (2010) UICC. TNM classification of malignant tumors, 7th edn. Springer, Berlin
Hamilton SR, Aaltonen LA (2000) Pathology and genetics. Tumours of the digestive system, 3rd edn. IARC Press, Lyon
Walker RA (2006) Quantification of immunohistochemistry—issues concerning methods, utility and semiquantitative assessment I. Histopathology 49(4):406–410. doi:10.1111/j.1365-2559.2006.02514.x
Alexander D, Hoffmann J, Munz A, Friedrich B, Geis-Gerstorfer J, Reinert S (2008) Analysis of OPLA scaffolds for bone engineering constructs using human jaw periosteal cells. J Mater Sci Mater Med 19(3):965–974. doi:10.1007/s10856-007-3351-8
von Rahden BH, Kircher S, Kafka M, Stuermer L, Reiber C, Gattenlohner S, Germer CT, Grimm M (2010) Glucocorticoid-induced TNFR family-related receptor (GITR)-expression in tumor infiltrating leucocytes (TILs) is associated with the pathogenesis of esophageal adenocarcinomas with and without Barrett’s mucosa. Cancer Biomark Sect A Dis Markers 7(6):285–294. doi:10.3233/CBM-2010-0192
Visvader JE, Lindeman GJ (2008) Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer 8(10):755–768. doi:10.1038/nrc2499
Nowell PC (1976) The clonal evolution of tumor cell populations. Science 194(4260):23–28
Campbell LL, Polyak K (2007) Breast tumor heterogeneity: cancer stem cells or clonal evolution? Cell Cycle 6(19):2332–2338. doi:10.4161/cc.6.19.4914
Yang C, Sudderth J, Dang T, Bachoo RM, McDonald JG, DeBerardinis RJ (2009) Glioblastoma cells require glutamate dehydrogenase to survive impairments of glucose metabolism or Akt signaling. Cancer Res 69(20):7986–7993. doi:10.1158/0008-5472.CAN-09-2266
Choo AY, Kim SG, Vander Heiden MG, Mahoney SJ, Vu H, Yoon SO, Cantley LC, Blenis J (2010) Glucose addiction of TSC null cells is caused by failed mTORC1-dependent balancing of metabolic demand with supply. Mol Cell 38(4):487–499. doi:10.1016/j.molcel.2010.05.007
Yin C, Qie S, Sang N (2012) Carbon source metabolism and its regulation in cancer cells. Crit Rev Eukaryot Gene Expr 22(1):17–35
Guppy M, Leedman P, Zu X, Russell V (2002) Contribution by different fuels and metabolic pathways to the total ATP turnover of proliferating MCF-7 breast cancer cells. Biochem J 364(Pt 1):309–315
Meng M, Chen S, Lao T, Liang D, Sang N (2010) Nitrogen anabolism underlies the importance of glutaminolysis in proliferating cells. Cell Cycle 9(19):3921–3932
Dang CV (2010) Glutaminolysis: supplying carbon or nitrogen or both for cancer cells? Cell Cycle 9(19):3884–3886
Wellen KE, Lu C, Mancuso A, Lemons JM, Ryczko M, Dennis JW, Rabinowitz JD, Coller HA, Thompson CB (2010) The hexosamine biosynthetic pathway couples growth factor-induced glutamine uptake to glucose metabolism. Genes Dev 24(24):2784–2799. doi:10.1101/gad.1985910
Yuneva M, Zamboni N, Oefner P, Sachidanandam R, Lazebnik Y (2007) Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J Cell Biol 178(1):93–105. doi:10.1083/jcb.200703099
Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT, Dang CV (2009) c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 458(7239):762–765. doi:10.1038/nature07823
Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon SB, Thompson CB (2008) Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci USA 105(48):18782–18787. doi:10.1073/pnas.0810199105
Hayry V, Makinen LK, Atula T, Sariola H, Makitie A, Leivo I, Keski-Santti H, Lundin J, Haglund C, Hagstrom J (2010) Bmi-1 expression predicts prognosis in squamous cell carcinoma of the tongue. Br J Cancer 102(5):892–897. doi:10.1038/sj.bjc.6605544
Perez-Sayans M, Suarez-Penaranda JM, Padin-Iruegas E, Gayoso-Diz P, Reis-De Almeida M, Barros-Angueira F, Gandara-Vila P, Blanco-Carrion A, Garcia-Garcia A (2014) Quantitative determination of c-myc facilitates the assessment of prognosis of OSCC patients. Oncol Rep 31(4):1677–1682. doi:10.3892/or.2014.3040
Shah NG, Trivedi TI, Tankshali RA, Goswami JV, Jetly DH, Shukla SN, Shah PM, Verma RJ (2009) Prognostic significance of molecular markers in oral squamous cell carcinoma: a multivariate analysis. Head Neck 31(12):1544–1556. doi:10.1002/hed.21126
Su Y, Simmen RC (2009) Soy isoflavone genistein upregulates epithelial adhesion molecule E-cadherin expression and attenuates beta-catenin signaling in mammary epithelial cells. Carcinogenesis 30(2):331–339. doi:10.1093/carcin/bgn279
Kobayashi N, Barnard RJ, Said J, Hong-Gonzalez J, Corman DM, Ku M, Doan NB, Gui D, Elashoff D, Cohen P, Aronson WJ (2008) Effect of low-fat diet on development of prostate cancer and Akt phosphorylation in the Hi-Myc transgenic mouse model. Cancer Res 68(8):3066–3073. doi:10.1158/0008-5472.CAN-07-5616
Du YP, Peng JS, Sun A, Tang ZH, Ling WH, Zhu HL (2009) Assessment of the effect of betaine on p16 and c-myc DNA methylation and mRNA expression in a chemical induced rat liver cancer model. BMC Cancer 9:261. doi:10.1186/1471-2407-9-261
Lu R, Wang X, Sun DF, Tian XQ, Zhao SL, Chen YX, Fang JY (2008) Folic acid and sodium butyrate prevent tumorigenesis in a mouse model of colorectal cancer. Epigenetics 3(6):330–335
Li C, Li M, Chen P, Narayan S, Matschinsky FM, Bennett MJ, Stanley CA, Smith TJ (2011) Green tea polyphenols control dysregulated glutamate dehydrogenase in transgenic mice by hijacking the ADP activation site. J Biol Chem 286(39):34164–34174. doi:10.1074/jbc.M111.268599
Griffiths M, Keast D, Patrick G, Crawford M, Palmer TN (1993) The role of glutamine and glucose analogues in metabolic inhibition of human myeloid leukaemia in vitro. Int J Biochem 25(12):1749–1755
Ahluwalia GS, Grem JL, Hao Z, Cooney DA (1990) Metabolism and action of amino acid analog anti-cancer agents. Pharmacol Ther 46(2):243–271
Duran RV, Oppliger W, Robitaille AM, Heiserich L, Skendaj R, Gottlieb E, Hall MN (2012) Glutaminolysis activates Rag-mTORC1 signaling. Mol Cell 47(3):349–358. doi:10.1016/j.molcel.2012.05.043
Duran RV, Hall MN (2012) Glutaminolysis feeds mTORC1. Cell Cycle 11(22):4107–4108. doi:10.4161/cc.22632
Kamata S, Kishimoto T, Kobayashi S, Miyazaki M, Ishikura H (2007) Possible involvement of persistent activity of the mammalian target of rapamycin pathway in the cisplatin resistance of AFP-producing gastric cancer cells. Cancer Biol Ther 6(7):1036–1043
Lee KH, Hur HS, Im SA, Lee J, Kim HP, Yoon YK, Han SW, Song SH, Oh DY, Kim TY, Bang YJ (2010) RAD001 shows activity against gastric cancer cells and overcomes 5-FU resistance by downregulating thymidylate synthase. Cancer Lett 299(1):22–28. doi:10.1016/j.canlet.2010.07.020
Yu CC, Hung SK, Liao HF, Lee CC, Lin HY, Lai HC, Li SC, Ho HC, Huang HB, Su YC (2014) RAD001 enhances the radiosensitivity of SCC4 oral cancer cells by inducing cell cycle arrest at the G2/M checkpoint. Anticancer Res 34(6):2927–2935
Duvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S, Vander Heiden MG, MacKeigan JP, Finan PM, Clish CB, Murphy LO, Manning BD (2010) Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell 39(2):171–183. doi:10.1016/j.molcel.2010.06.022
Zha X, Sun Q, Zhang H (2011) mTOR upregulation of glycolytic enzymes promotes tumor development. Cell Cycle 10(7):1015–1016
Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C, Myer VE, MacKeigan JP, Porter JA, Wang YK, Cantley LC, Finan PM, Murphy LO (2009) Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 136(3):521–534. doi:10.1016/j.cell.2008.11.044
Shukla SK, Gebregiworgis T, Purohit V, Chaika NV, Gunda V, Radhakrishnan P, Mehla K, Pipinos II, Powers R, Yu F, Singh PK (2014) Metabolic reprogramming induced by ketone bodies diminishes pancreatic cancer cachexia. Cancer Metab 2:18. doi:10.1186/2049-3002-2-18
Grimm M (2012) Prognostic value of clinicopathological parameters and outcome in 484 patients with oral squamous cell carcinoma: microvascular invasion (V+) is an independent prognostic factor for OSCC. Clin Transl Oncol 14(11):870–880. doi:10.1007/s12094-012-0867-2
Perez-Sayans M, Suarez-Penaranda JM, Pilar GD, Barros-Angueira F, Gandara-Rey JM, Garcia-Garcia A (2011) Hypoxia-inducible factors in OSCC. Cancer Lett 313(1):1–8. doi:10.1016/j.canlet.2011.08.017
Wu MC, Arimura GK, Yunis AA (1978) Mechanism of sensitivity of cultured pancreatic carcinoma to asparaginase. Int J Cancer Journal international du cancer 22(6):728–733
Narta UK, Kanwar SS, Azmi W (2007) Pharmacological and clinical evaluation of l-asparaginase in the treatment of leukemia. Crit Rev Oncol Hematol 61(3):208–221. doi:10.1016/j.critrevonc.2006.07.009
Avramis VI, Panosyan EH (2005) Pharmacokinetic/pharmacodynamic relationships of asparaginase formulations: the past, the present and recommendations for the future. Clin Pharmacokinet 44(4):367–393. doi:10.2165/00003088-200544040-00003
Lessner HE, Valenstein S, Kaplan R, DeSimone P, Yunis A (1980) Phase II study of l-asparaginase in the treatment of pancreatic carcinoma. Cancer Treat Rep 64(12):1359–1361
Enns GM, Berry SA, Berry GT, Rhead WJ, Brusilow SW, Hamosh A (2007) Survival after treatment with phenylacetate and benzoate for urea-cycle disorders. N Engl J Med 356(22):2282–2292. doi:10.1056/NEJMoa066596
Thibault A, Samid D, Cooper MR, Figg WD, Tompkins AC, Patronas N, Headlee DJ, Kohler DR, Venzon DJ, Myers CE (1995) Phase I study of phenylacetate administered twice daily to patients with cancer. Cancer 75(12):2932–2938
Thibault A, Cooper MR, Figg WD, Venzon DJ, Sartor AO, Tompkins AC, Weinberger MS, Headlee DJ, McCall NA, Samid D et al (1994) A phase I and pharmacokinetic study of intravenous phenylacetate in patients with cancer. Cancer Res 54(7):1690–1694
Ploessl K, Wang L, Lieberman BP, Qu W, Kung HF (2012) Comparative evaluation of 18F-labeled glutamic acid and glutamine as tumor metabolic imaging agents. J Nucl Med 53(10):1616–1624. doi:10.2967/jnumed.111.101279
Acknowledgments
We thank Julia Grimm for her technical assistance.
Conflict of interest
The authors have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cetindis, M., Biegner, T., Munz, A. et al. Glutaminolysis and carcinogenesis of oral squamous cell carcinoma. Eur Arch Otorhinolaryngol 273, 495–503 (2016). https://doi.org/10.1007/s00405-015-3543-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-015-3543-7