Abstract
Purpose
Postpartum depression (PPD) represents a significant challenge to maternal and child health. Early screening for PPD is essential to ensure appropriate treatment and support. The present study aimed to assess whether maternal prepartum anaemia influences the likelihood of developing PPD within 3 days after delivery.
Methods
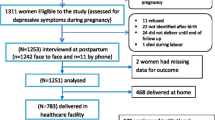
In collaboration with the Department of Psychiatry, a prospective observational study was carried out at the Gynaecology and Obstetrics Department of the University of Campania “Luigi Vanvitelli” in Naples. A total of 211 full-term pregnant women were enrolled, and their predelivery haemoglobin value was recorded. Women with gestational diabetes, hypertension, pre-eclampsia, intrauterine growth restriction, intellectual disability, or pre-existing diagnosis of psychotic spectrum disorder were excluded. Participants provided written informed consent to fill out the Edinburgh Postnatal Depression Scale (EPDS) 3 days after delivery. EPDS cut-off score of ≥ 10 was used to identify women at risk of developing PPD. Statistical analysis was performed using Student's t test, the Wilcoxon Rank Sum test, and linear regression.
Results
The participants were categorized into 2 groups based on EPDS scores: EPDS < 10 (176 patients) or EPDS ≥ 10 (35 patients). The two groups showed homogeneity in terms of socio-demographic and clinical characteristics. The mean haemoglobin values of anaemic pregnant women in the EPDS ≤ 10 group (11.78 ± 1.39 g/dl) and the EPDS > 10 group (11.62 ± 1.27 g/dl) were not significantly different (p = 0.52). There was no significant correlation between the predelivery haemoglobin value and the EPDS postpartum score of < 10 or ≥ 10. The Wilcoxon Rank Sum test and the estimated coefficients of the linear regression model did not show any statistical relationship between continuous and binary haemoglobin values.
Conclusions
Our study found that maternal prepartum anaemia did not negatively impact the likelihood of developing postpartum depressive symptoms, in the first 3 days after delivery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This study contributes to current knowledge by providing evidence that prepartum anaemia does not increase the likelihood of developing postpartum depression within three days of delivery, nor does it influence the anhedonia, anxiety, and depression subscales of the Edinburgh Postnatal Depression Scale. Our findings contribute to clinical practice by establishing a basis for future investigations into the potential correlation between pregnancy anaemia and postpartum depression. |
Introduction
Anaemia is one of the most significant global public health issues impacting people's physical and mental abilities [1]. Geography, lifestyle, and diet influence the prevalence of anaemia in pregnant women, estimated to range between 14 and 80% in various countries [2,3,4,5]. Recently, consideration has been given to the function of iron deficiency anaemia (IDA). Women who have had IDA during pregnancy are more likely to have a delayed recovery of iron reserves in the postpartum period. The prevalence of IDA during pregnancy is around 7.5% [6, 7]. Behavioural symptoms associated with anaemia in adults include changes in cognition, emotions, irritability, apathy, fatigue, depressive symptoms, and hypoactivity [7, 8]. Even though anaemia during pregnancy is more prevalent in countries with low or middle incomes than in countries with high incomes, the prevalence of anaemia in these countries is still high, especially in Asia [9]. In Europe, the prevalence of IDA is 21–35% [10]. Several studies have demonstrated that anaemia plays a role in the development of adverse pregnancy outcomes, such as pre-eclampsia, premature rupture of membranes, low birth weight, preterm birth, and fetal and maternal mortality [11,12,13]. Because anaemia may produce weariness, irritability, lethargy, and depressive symptoms during pregnancy or the postpartum period has been identified as a possible physiological risk factor for PPD (PPD) [5, 9, 14]. The potential mechanisms by which anaemia causes depressive symptoms are generally explained by altering myelinisation and neurotransmitter metabolism [15].
PPD typically occurs between 6 and 12 weeks postpartum [16, 17]. Depending on the diagnostic criteria and screening instruments used, the prevalence of PPD ranges between 10 and 15% [18]. In addition to affecting the mother’s mental health, it disrupts family relationships and the child’s emotional and cognitive development [19,20,21,22,23,24,25]. PPD is a major risk factor for suicide among postpartum mothers worldwide [26, 27], requiring a pharmacological intervention [25] apart from psychological interventions usually provided to women with PPD [28]. Several psychological risk factors have been identified in PPD, including a previous history of depression, elevated levels of postnatal stress, and inadequate social and financial support during the postpartum period [29,30,31,32,33]. However, limited research has been conducted on physiological variables that may contribute to the development of PPD [34] and currently, it is not known whether this disorder can be considered a clinical entity psychopathologically different from Major Depressive Disorder [35, 36]. The Edinburgh Postnatal Depression Scale (EPDS) is a widely regarded screening tool for detecting postpartum depression [37], despite other instruments are available [38]. The EPDS effectively identifies, with good sensitivity and specificity, women susceptible to developing PPD [39]. In the 1980s, John Cox, Jeni Holden, and Ruth Sagovsky developed the EPDS in Edinburgh, Scotland [40]. The authors aimed to develop a tool capable of detecting symptoms of depression and identifying the severe and persistent ones that require clinical intervention [40]. The EPDS is typically administered on the third day after delivery and repeated at subsequent follow-up appointments [41]. The mother self-administered the questionnaire, which should take less than 5 min to complete [42]. EPDS consists of 10 items that assess the presence and severity of symptoms of depression. The items are scored on a four-point scale ranging from 0 to 3, with scores ranging from 0 to 30 [43]. The EPDS total score is calculated by summing the scores of each item, with higher scores indicating greater severity of depressive symptoms [44]. The recommended cut-off points for the EPDS vary depending on the study design and population being examined [45]. A score of 13 or above is suggested to indicate severe depressive symptoms, but a score of 10 or above is commonly used to screen for PPD [46]. The sensitivity and specificity of different cut-off points vary, and local prevalence rates and available resources should inform the choice of cut-off point [45, 47]. However, it is essential to note that the EPDS is a screening tool, not a diagnostic instrument [48]. A healthcare professional should further evaluate any woman who scores positively on the EPDS, and clinical judgment is necessary to determine the presence and severity of postnatal depression [49]. In this prospective study, we used a validated depression score (EPDS and EPDS subscales) to explore the occurrence of prepartum anaemia and its potential correlation with PPD. Timely screening for postnatal depression is essential to ensure appropriate treatment and support for affected women. Identifying and managing PPD promptly is crucial in order to prevent adverse outcomes. Early recognition of a risk factor such as prepartum anaemia is crucial for promoting the mental health of both mothers and their children.
Materials and methods
The present study was a longitudinal observational study conducted at the Gynaecology and Obstetrics Department of the University of Campania “Luigi Vanvitelli” in Naples, in collaboration with the Department of Psychiatry. All full-term pregnant admitted to our institution’s delivery room between December 2019 and February 2021 were invited to participate. We excluded women with a severe intellectual disability or a pre-existing diagnosis of schizophrenia, schizoaffective disorder, delusional disorder, bipolar or other unspecified psychotic spectrum disorder. Furthermore, in order to achieve a homogeneous obstetric population and avoid bias, we excluded pregnancies complicated by gestational and pregestational diabetes, chronic hypertension, gestational hypertension, pre-eclampsia/eclampsia, intrauterine fetal growth restriction, preterm delivery, multiple pregnancies and pregnancies with fetal abnormalities detected prenatally. The participants were enrolled before the onset of labor and provided their written informed consent after understanding the study’s objectives. Following the delivery, precisely 3 days postpartum, participants were instructed to fill out the EPDS Italian version [50]. Upon enrollment, the following information was collected for all pregnant women: (1) socio-demographic characteristics (age, level of education, marital and employment status); (2) clinical information on pregnancy in progress (spontaneous previous abortions, vaginal delivery vs caesarean, presence of obstetric complications during pregnancy including eclampsia and gestational diabetes, the Apgar index at the 1st and 5 min); (4) social and contextual factors (relationship with the partner, family conflicts, socio-economic situation); (5) all data regarding the blood chemistry tests performed at the time of admission. All patients carry out the usual routine blood chemistry tests provided by our facility at hospitalization in accordance with good medical practices. The haemoglobin value obtained from the hospitalisation tests was utilised as an indicator for evaluating the existence of maternal anaemia. As per the definition provided by the World Health Organization (WHO), the diagnosis of anaemia in the third trimester of pregnancy is established when the concentration of haemoglobin falls below 11.0 g/dL [51]. As previously mentioned, we used the EPDS on the third day after delivery to evaluate depressive symptoms in the postpartum period. The EPDS is a validated questionnaire developed to detect postnatal depression. The questionnaire consists of ten self-reported items rated on a four-point scale. The EPDS has been found to have good sensitivity and specificity, making it effective in identifying postnatal depression onset. Various cut-off points have been determined in validation studies [45]. A score of 13 or above is suggested to indicate severe depressive symptoms, but a score of 10 or above is commonly used for screening postnatal depression [46]. In this study, we used a cut-off ≥ 10 to identify women at increased risk of developing PPD [37]. Three subscales were extracted from the EPDS [52], namely the anhedonia subscale (items 1 and 2), the anxiety subscale (items 3 to 6) and the depression subscale (item 7 to 10). This study was conducted following globally accepted standards of good clinical practice, in accordance with the Declaration of Helsinki and with national and local regulations. The study protocol was approved by the Ethics Committee of the University of Campania “Luigi Vanvitelli” (protocol number 98 of February 28, 2019).
Statistical analysis
Continuous variables were reported as either the means and standard deviation or median and interquartile ranges (IQRs) according to their distribution, as assessed by the Shapiro–Wilk normality test. Categorical variables were reported as percentages. Differences in characteristics of patients between EPDS groups were tested by t-test or Wilcoxon rank sum test (according to their distribution) and Pearson chi-squared or Fisher's exact test (according to their distribution) for continuous and categorical variables, respectively. To measure the linear association between continuous variables, Pearson correlation test was used if variables had a normal distribution, otherwise Spearman’s rank correlation test was calculated.
To measure the effect of the HGB on the EPDS, four regression models were used. In particular, two linear regression model were estimated using the EPDS scale as continuous response variable and HGB as predictor variable in continuous and binary form, respectively; moreover, two logistic regression model were estimated using the categorized EPDS as binary response variable and the same predictors (i.e., continuous and categorized HGB variable). All the regression coefficients were adjusted for several socio-demographic, clinical and contextual baseline patients’ characteristics.
All the statistical analyses were performed by the R Studio Statistical software, version 4.1.3.
Results
A total of 234 pregnant women met the inclusion criteria. All participants were invited to take part, however, only 211 individuals agreed to participate. All enrolled patients’ haemoglobin (Hb) values were obtained from the routine admission venous blood sample. The participants were categorised into 2 groups based on the EPDS ≤ 10 (176 patients) or > 10 (35 patients). Based on the Hb status, EPDS ≤ 10 group included 8 anaemic pregnant versus 47 anaemic pregnant of EPDS > 10 group (mean Hb values of 11.78 ± 1.39 g/dl versus 11.62 ± 1.27 g/dl, p value 0.52). Hence, it can be observed that the two groups show homogeneity in terms of age, education, prior BMI, and gestational age at delivery, employment and Apgar score, as no statistical differences were found when comparing these variables (Table 1). The women enrolled in the study had a median age of 32.00 (± 7.75) years. Additionally, a significant proportion of these women (40.6%) possessed a good level of education (Table 1). No statistical distinctions were observed between the two groups regarding cigarette consumption patterns (8.5% were smokers—Table 1). No significant differences were observed in the clinical information regarding current pregnancy, as well as the social and contextual aspects, between the two groups. These factors included the (relationship with the partner, family conflicts and socio-economic situation (Table 1). A Scatter-plot of the Hb level and EPDS scale stratified for EPDS groups is shown in Fig. 1. A postpartum EPDS score of ≤ 10 or > 10 did not correlate significantly with the predelivery Hgb value (Table 2). We have also employed the Wilcoxon Rank Sum test on Hb levels and EPDS groups to investigate the haemoglobin levels of the pregnant women in the two study groups. Figure 2 presents the box plot comparison of the two data sets. The Wilcoxon Rank Sum test did not indicate a statistical relationship. The estimated coefficients of the linear regression model with continuous and binary HGB values indicated the absence of a statistical relationship (Tables 3, 4 and 5). The sub-analysis of the EPDS subscales also did not show significant statistical differences between pregnant with or without anaemia (Table 6).
Discussion
The scientific community is debating different risk factors associated with an increased likelihood of developing PPD. The precise identification of risk factors associated with the development of PPD prior to delivery could significantly support physicians in managing this commonly underestimated condition, and to tailor treatments for women with depressive symptoms during the postpartum period [53], also il line with women treatments preferences [54]. The relationship between anaemia during the third trimester of pregnancy and PPD remains unclear due to conflicting results in various studies. Additionally, no research has focused on the connection between maternal anaemia and the subscales of the EPDS. Our research revealed no link between prepartum anaemia and an increased probability of developing symptoms of PPD within three days after delivery. Furthermore, our analysis determined that the EPDS subscales did not correlate with prepartum anaemia. A recent meta-analysis of 15 studies examines the connection between anaemia and the risk of maternal depression [55]. The findings demonstrate a significant association between anaemia and an increased risk of maternal depression, with an odds ratio of 1.53 [55]. However, of the included studies, only seven assessed postpartum symptoms. Notably, Albacar et al. evaluated depressive symptoms and obtained blood samples 48 h after delivery, focusing only on iron storage levels and the inflammatory marker [56]. Alharbi et al. [57], through an observational case–control and retrospective study, revealed a correlation between pregnancy anaemia and EPDS after 8–12 weeks postpartum in Saudi Arabia. Chandrasekaran et al. specifically examined the relationship between PPD and postpartum anaemia only in women who underwent elective caesarean sections [58]. Eckerdal et al. investigated the impact of heavy postpartum haemorrhage on PPD onset [59]. Another research conducted by Azita Goshtasebi et al. explored the influence of prepartum haemoglobin levels on PPD among urban pregnant women in Iran [60]. This study suggests that a haemoglobin level below 11 g/dL at delivery may increase the likelihood of experiencing PPD (EPDS cut-off adopted > 13). It should be noted that this finding is specific to haemoglobin levels and not necessarily related to iron deficiency. The study sample included healthy women at low risk for complications, and none of the women in this study had iron deficiency anaemia at delivery (low haemoglobin and ferritin values) [60]. Surkan et al. [61] assessed PPD in a low-income country using their own scale, adapted from the Patient Health Questionnaire (PHQ-9) and the Center for Epidemiologic Studies Depression Scale (CES-D). Xu et al. [62] examined hospitalization rates for anaemia and depression in women three years before and three years after birth. It is crucial to highlight that the studies mentioned in the meta-analysis are heterogeneous and differ in terms of scales, EPDS cut-off points, and timing for postpartum EPDS administration when examining the correlation between PPD and maternal anaemia [55]. In another recent study, Maeda et al. administered EPDS four weeks after the delivery and the blood test was performed in the second and third trimesters and one week after the delivery. The authors, with an EPDS cut-off of 9, demonstrated how low Hb levels in the first week of the postpartum period were significantly associated with an increased PPD risk, while no such correlation was observed between anaemia or Hb levels during the second and third trimesters and PPD risk [9]. Corwin et al. analyzed 37 women and reported how the low haemoglobin level seven days after delivery was negatively correlated with self-reported depressive symptoms at day 28 [6]. Corwin et al. adopted the Center for Epidemiological Studies-Depressive Symptomatology Scale (CES-D) to screen for depressive symptoms.
The precise connection between prepartum anaemia and PPD is not completely clear, and one possibility is that postpartum anaemia causes behavioural and psychological symptoms similar to those induced by iron deficiency anaemia: it is a form of anaemia caused by iron deficiency, and its mechanism differs from that of second and third-trimester anaemia, which may occur due to a physiological increase in plasma volume (hemodilution) [9]. Our findings, for the first time, demonstrated how prepartum anaemia does not modify the likelihood of developing PPD in the immediate postpartum period. Simultaneously, we observed that anaemia did not influence the EPDS subscale, which includes the anhedonia, anxiety, and depression subscales. The study possesses several notable strengths. Firstly, it adopts a prospective design with a prospective collection of data. Additionally, the study benefits from a large population. Moreover, the data collection adopted a scientifically validated assessment tool, the EPDS, administered within three days after the delivery. It is worth noting that the EPDS screening for PPD, and the immediate postpartum period were not previously correlated to peripartum anaemia. Moreover, to attain a uniform obstetric population, we specifically recruited individuals with uncomplicated pregnancies, excluding those with gestational and pregestational pathologies: gestational and pregestational diabetes, chronic hypertension, gestational hypertension, pre-eclampsia/eclampsia, intrauterine fetal growth restriction, preterm delivery, multiple pregnancies and pregnancies with fetal abnormalities detected prenatally. At least the diagnosis of anaemia was performed homogeneously and systematically in our hospital laboratory. However, it is essential to acknowledge the limitations of our study. Firstly, there may be methodological limitations in our estimation of PPD prevalence as we just relied on the EPDS screening tool to assess depressive symptoms. A definitive diagnosis of PPD can only be made through a structured psychiatric interview, which we offered to all patients who tested positive for EPDS. Second, due to the exclusive collection of haemoglobin data during hospitalization, the correlation between anaemia during each trimester of pregnancy and the EPDS results was unavailable. Third, we have not collected data on iron, vitamin B12, folic acid levels, and other factors that cause maternal anaemia. Nevertheless, additional research is required to elucidate the potential implications of anaemia on the subsequent postpartum period (after three days of delivery). Furthermore, clinical trials are necessary to assess the efficacy of anaemia treatment during pregnancy in mitigating the risk of PPD.
Conclusion
According to our study's findings, maternal prepartum anaemia did not negatively impact the likelihood of developing postpartum depressive symptoms, as measured by the EPDS, in the first three days following the delivery.
Data Availability
The data that support the findings of this study are not publicly available due to privacy reasons but are available from the corresponding author upon reasonable request.
References
Azami M, Badfar G, Khalighi Z, Qasemi P, Shohani M, Soleymani A et al (2019) The association between anemia and postpartum depression: a systematic review and meta-analysis. Caspian J Intern Med 10(2):115
Azami M, Darvishi Z, Sayehmiri K (2016) Systematic review and meta-analysis of the prevalence of anemia among pregnant Iranian women (2005–2015). Shiraz E-Med J 17(4–5)
Sayehmiri K, Darvishi Z, Azami M, Qavam S (2015) The prevalence of anemia in first, second and third trimester of pregnancy in Iran: a systematic review and meta-analysis. Iran J Obstet Gynecol Infertil 18(168):7–15
Stevens GA, Finucane MM, De-Regil LM, Paciorek CJ, Flaxman SR, Branca F et al (2013) Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. Lancet Glob Health 1(1):e16–e25
Correll CU, Solmi M, Croatto G, Schneider LK, Rohani-Montez SC, Fairley L et al (2022) Mortality in people with schizophrenia: a systematic review and meta-analysis of relative risk and aggravating or attenuating factors. World Psychiatry 21(2):248–271
Corwin EJ, Murray-Kolb LE, Beard JL (2003) Low hemoglobin level is a risk factor for postpartum depression. J Nutr 133(12):4139–4142
Murray-Kolb LE, Beard JL (2009) Iron deficiency and child and maternal health. Am J Clin Nutr 89(3):946S-S950
Ludwig H, Strasser K (eds) (2001) Symptomatology of anemia. Seminars in oncology. Elsevier
Maeda Y, Ogawa K, Morisaki N, Tachibana Y, Horikawa R, Sago H (2020) Association between perinatal anemia and postpartum depression: a prospective cohort study of Japanese women. Int J Gynecol Obstet 148(1):48–52
Milman N, Taylor CL, Merkel J, Brannon PM (2017) Iron status in pregnant women and women of reproductive age in Europe. Am J Clin Nutr 106(suppl_6):1655S–62S
Badfar G, Shohani M, Soleymani A, Azami M (2019) Maternal anemia during pregnancy and small for gestational age: a systematic review and meta-analysis. J Matern Fetal Neonatal Med 32(10):1728–1734
Rahmati S, Delpishe A, Azami M, Ahmadi MRH, Sayehmiri K (2017) Maternal Anemia during pregnancy and infant low birth weight: a systematic review and meta-analysis. Int J Reprod Biomed 15(3):125
Rahmati S, Azami M, Badfar G, Parizad N, Sayehmiri K (2020) The relationship between maternal anemia during pregnancy with preterm birth: a systematic review and meta-analysis. J Matern Fetal Neonatal Med 33(15):2679–2689
Freedman R, Hunter SK, Law AJ, Hoffman MC (2021) Prenatal prevention of psychiatric illness and childhood development population-wide. World Psychiatry 20(2):226
Beard JL, Connor JR (2003) Iron status and neural functioning. Annu Rev Nutr 23(1):41–58
Doucet S, Dennis C-L, Letourneau N, Blackmore ER (2009) Differentiation and clinical implications of postpartum depression and postpartum psychosis. J Obstet Gynecol Neonatal Nurs 38(3):269–279
Leung BM, Kaplan BJ (2009) Perinatal depression: prevalence, risks, and the nutrition link—a review of the literature. J Am Diet Assoc 109(9):1566–1575
Marcus SM (2009) Depression during pregnancy: rates, risks and consequences. J Popul Ther Clin Pharmacol 16(1).
Gjerdingen DK, Yawn BP (2007) Postpartum depression screening: importance, methods, barriers, and recommendations for practice. J Am Board Family Med 20(3):280–288
Wójcik J, Dudek D, Schlegel-Zawadzka MG, Grabowska M, Marcinek A, Florek E et al (2006) Antepartum/postpartum depressive symptoms and serum zinc and magnesium levels. Pharmacol Reports 58(4):571
Lång U, Ramsay H, Yates K, Veijola J, Gyllenberg D, Clarke MC et al (2022) Potential for prediction of psychosis and bipolar disorder in Child and Adolescent Mental Health Services: a longitudinal register study of all people born in Finland in 1987. World Psychiatry 21(3):436–443
Treyvaud K, Brown SJ (2022) Mental health of children and parents after very preterm birth. World Psychiatry 21(1):148
Wang Z, Chan AY, Ho PW, Wong KH, Brauer R, Besag FM et al (2022) Prenatal exposure to antidepressants or antipsychotics and the risk of seizure in children. World Psychiatry 21(2):322–323
Andresen JB, Graugaard C, Andersson M, Bahnsen MK, Frisch M (2022) Adverse childhood experiences and mental health problems in a nationally representative study of heterosexual, homosexual and bisexual Danes. World Psychiatry 21(3):427–435
Galbally M, Woods N, Snellen M (2022) How clinicians can support women in making decisions about psychopharmacological treatments in pregnancy. World Psychiatry 21(1):149
Fuhr DC, Calvert C, Ronsmans C, Chandra PS, Sikander S, De Silva MJ et al (2014) Contribution of suicide and injuries to pregnancy-related mortality in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Psychiatry 1(3):213–225
Turner BJ (2022) Detecting and managing non-suicidal self-damaging behaviors. World Psychiatry 21(3):461
Hayes SC, Hofmann SG (2021) “Third-wave” cognitive and behavioral therapies and the emergence of a process-based approach to intervention in psychiatry. World Psychiatry 20(3):363–375
Norhayati M, Hazlina NN, Asrenee A, Emilin WW (2015) Magnitude and risk factors for postpartum symptoms: a literature review. J Affect Disord 175:34–52
Luciano M, Di Vincenzo M, Brandi C, Tretola L, Toricco R, Perris F et al (2022) Does antenatal depression predict post-partum depression and obstetric complications? Results from a longitudinal, long-term, real-world study. Front Psych 13:1082762
Pearlstein T, Howard M, Salisbury A, Zlotnick C (2009) Postpartum depression. Am J Obstet Gynecol 200(4):357–364
Righetti-Veltema M, Conne-Perréard E, Bousquet A, Manzano J (1998) Risk factors and predictive signs of postpartum depression. J Affect Disord 49(3):167–180
de Mendonça Lima CA, De Leo D, Ivbijaro G, Svab I (2021) Loneliness and abuse as risk factors for suicide in older adults: new developments and the contribution of the WPA Section on Old Age Psychiatry. World Psychiatry 20(3):455
Field T (2017) Prenatal depression risk factors, developmental effects and interventions: a review. J Pregnancy Child Health 4(1)
Lahey BB, Moore TM, Kaczkurkin AN, Zald DH (2021) Иepapxичecкиe мoдeли пcиxoпaтoлoгии: эмпиpичecкaя пoддepжкa, пocлeдcтвия и нepeшeнныe вoпpocы. World Psychiatry 20(1):57–63
Watson D, Levin-Aspenson HF, Waszczuk MA, Conway CC, Dalgleish T, Dretsch MN et al (2022) Validity and utility of Hierarchical Taxonomy of Psychopathology (HiTOP): III. Emotional dysfunction superspectrum. World Psychiatry 21(1):26–54
Levis B, Negeri Z, Sun Y, Benedetti A, Thombs BD (2020) Accuracy of the Edinburgh Postnatal Depression Scale (EPDS) for screening to detect major depression among pregnant and postpartum women: systematic review and meta-analysis of individual participant data. BMJ 371
Kroenke K (2021) PHQ-9: global uptake of a depression scale. World Psychiatry 20(1):135
Thombs BD, Benedetti A, Kloda LA, Levis B, Riehm KE, Azar M et al (2015) Diagnostic accuracy of the Edinburgh Postnatal Depression Scale (EPDS) for detecting major depression in pregnant and postnatal women: protocol for a systematic review and individual patient data meta-analyses. BMJ Open 5(10):e009742
Cox JL, Holden JM, Sagovsky R (1987) Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry 150(6):782–786
Rubertsson C, Börjesson K, Berglund A, Josefsson A, Sydsjö G (2011) The Swedish validation of Edinburgh postnatal depression scale (EPDS) during pregnancy. Nord J Psychiatry 65(6):414–418
Horowitz JA, Murphy CA, Gregory KE, Wojcik J (2011) A community-based screening initiative to identify mothers at risk for postpartum depression. J Obstet Gynecol Neonatal Nurs 40(1):52–61
Beck CT, Gable RK (2001) Comparative analysis of the performance of the Postpartum Depression Screening Scale with two other depression instruments. Nurs Res 50(4):242–250
Verreault N, Da Costa D, Marchand A, Ireland K, Dritsa M, Khalifé S (2014) Rates and risk factors associated with depressive symptoms during pregnancy and with postpartum onset. J Psychosom Obstet Gynecol 35(3):84–91
Gibson J, McKenzie-McHarg K, Shakespeare J, Price J, Gray R (2009) A systematic review of studies validating the Edinburgh Postnatal Depression Scale in antepartum and postpartum women. Acta Psychiatr Scand 119(5):350–364
Hewitt CE, Gilbody SM, Mann R, Brealey S (2010) Instruments to identify post-natal depression: which methods have been the most extensively validated, in what setting and in which language? Int J Psychiatry Clin Pract 14(1):72–76
Luciano M, Sampogna G, Del Vecchio V, Giallonardo V, Perris F, Carfagno M et al (2021) The transition from maternity blues to full-blown perinatal depression: results from a longitudinal study. Front Psychiatry 12
Matijasevich A, Munhoz TN, Tavares BF, Barbosa APPN, da Silva DM, Abitante MS et al (2014) Validation of the Edinburgh Postnatal Depression Scale (EPDS) for screening of major depressive episode among adults from the general population. BMC Psychiatry 14(1):1–9
Wisner KL, Sit DK, McShea MC, Rizzo DM, Zoretich RA, Hughes CL et al (2013) Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. JAMA Psychiat 70(5):490–498
Benvenuti P, Ferrara M, Niccolai C, Valoriani V, Cox JL (1999) The Edinburgh postnatal depression scale: validation for an Italian sample. J Affect Disord 53(2):137–141
Garcia-Casal MN, Pasricha SR, Sharma AJ, Peña-Rosas JP (2019) Use and interpretation of hemoglobin concentrations for assessing anemia status in individuals and populations: results from a WHO technical meeting. Ann N Y Acad Sci 1450(1):5–14
Tuohy A, McVey C (2008) Subscales measuring symptoms of non-specific depression, anhedonia, and anxiety in the Edinburgh Postnatal Depression Scale. Br J Clin Psychol 47(2):153–169
Davidson L, Tondora J (2022) Person-centred care planning as foundational to clinical practice. World Psychiatry 21(1):1
Swift JK, Mullins RH, Penix EA, Roth KL, Trusty WT (2021) The importance of listening to patient preferences when making mental health care decisions. World Psychiatry 20(3):316
Kang SY, Kim H-B, Sunwoo S (2020) Association between anemia and maternal depression: a systematic review and meta-analysis. J Psychiatr Res 122:88–96
Albacar G, Sans T, Martín-Santos R, García-Esteve L, Guillamat R, Sanjuan J et al (2011) An association between plasma ferritin concentrations measured 48 h after delivery and postpartum depression. J Affect Disord 131(1–3):136–142
Alharbi AA, Abdulghani HM (2014) Risk factors associated with postpartum depression in the Saudi population. Neuropsychiatr Dis Treat 311–316
Chandrasekaran N, De Souza LR, Urquia ML, Young B, Mcleod A, Windrim R et al (2018) Is anemia an independent risk factor for postpartum depression in women who have a cesarean section?-A prospective observational study. BMC Pregnancy Childbirth 18:1–7
Eckerdal P, Kollia N, Löfblad J, Hellgren C, Karlsson L, Högberg U et al (2016) Delineating the association between heavy postpartum haemorrhage and postpartum depression. PLoS One 11(1):e0144274
Goshtasebi A, Alizadeh M, Gandevani SB (2013) Association between maternal anaemia and postpartum depression in an urban sample of pregnant women in Iran. J Health Popul Nutr 31(3):398
Surkan PJ, Sakyi KS, Christian P, Mehra S, Labrique A, Ali H et al (2017) Risk of depressive symptoms associated with morbidity in postpartum women in rural Bangladesh. Matern Child Health J 21:1890–1900
Xu F, Roberts L, Binns C, Sullivan E, Homer CS (2018) Anaemia and depression before and after birth: a cohort study based on linked population data. BMC Psychiatry 18(1):1–12
Funding
Open access funding provided by Università degli Studi della Campania Luigi Vanvitelli within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
MLV, MT, ML and MDV: project development, DL and MLV: manuscript writing/editing, MP, MMM, CB, MDV, MC: data collection or management, MF: data analysis, ML, MDV, CB: protocol/project development, GS: project development,
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study protocol was approved by the Ethics Committee of the University of Campania “Luigi Vanvitelli”, Naples (approval number: protocol number 98 of February 28, 2019).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
La Verde, M., Luciano, M., Fordellone, M. et al. Is there a correlation between prepartum anaemia and an increased likelihood of developing postpartum depression? A prospective observational study. Arch Gynecol Obstet (2024). https://doi.org/10.1007/s00404-023-07344-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00404-023-07344-7