Abstract
Purpose
To examine the association between endometriosis and adverse pregnancy and perinatal outcomes (preeclampsia, placenta previa, and preterm birth).
Methods
A population-based retrospective cohort study was conducted among 468,778 eligible women who contributed 912,747 singleton livebirths between 1980 and 2015 in Western Australia (WA). We used probabilistically linked perinatal and hospital separation data from the WA data linkage system’s Midwives Notification System and Hospital Morbidity Data Collection databases. We used a doubly robust estimator by combining the inverse probability weighting with the outcome regression model to estimate adjusted risk ratios (RR) and 95% confidence intervals (CIs).
Results
There were 19,476 singleton livebirths among 8874 women diagnosed with endometriosis. Using a doubly robust estimator, we found pregnancies in women with endometriosis to be associated with an increased risk of preeclampsia with RR of 1.18, 95% CI 1.11–1.26, placenta previa (RR 1.59, 95% CI 1.42–1.79) and preterm birth (RR 1.45, 95% CI 1.37–1.54). The observed association persisted after stratified by the use of Medically Assisted Reproduction, with a slightly elevated risk among pregnancies conceived spontaneously.
Conclusions
In this large population-based cohort, endometriosis is associated with an increased risk of preeclampsia, placenta previa, and preterm birth, independent of the use of Medically Assisted Reproduction. This may help to enhance future obstetric care among this population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Women with endometriosis have a greater risk of preeclampsia, placenta previa, and preterm birth, which cannot be explained by the use of medically assisted reproduction. These findings may inform future obstetric care among this population. |
Introduction
Endometriosis is a chronic inflammatory condition affecting women, where endometrial cells normally lining up the uterine cavity are found outside the uterus. Endometriosis can cause a variety of and sometimes unspecific symptoms with no to severe cyclic pain episodes, dyspareunia, dysmenorrhea, and subfertility [1, 2]. The disease highly affects the quality of life, and productivity, and causes high treatment and societal costs [3]. It often takes 8–12 years from symptom onset to surgical diagnosis [4,5,6], leading to varying prevalence estimates (5–50% in infertile women, up to 75% in cases with chronic pain) [7, 8]. In Australia, 11% of reproductive-age women are affected with prevalence ranging from 2 to 11% in asymptomatic women [9]. Three-quarters of women with mild to moderate endometriosis can achieve pregnancy spontaneously, despite an increased risk of subfertility [10].
The association between endometriosis and adverse pregnancy outcomes has drawn more attention in recent years with fairly consistent evidence of increased risks for caesarean section, preterm birth, and stillbirth [11, 12]. However, the link with gestational diabetes, preeclampsia, or intrauterine growth restriction remains less clear due to heterogeneity in study designs and methodologies used in previous studies [13,14,15,16,17,18,19,20,21]. In epidemiology, it remains challenging to study the direct impact of endometriosis on pregnancy outcomes, and underlying mechanisms are not well understood. Much of the existing research on this topic comes from small cohort studies at infertility clinics or single surgical centres [22], which can produce results that are misinterpreted as evidence of no association rather than a lack of evidence for any association. Moreover, data limited to clinical settings are prone to selection bias as these participants may have better access to care, which may be linked to other health behaviours that affect pregnancy outcomes [23]. Classical study designs adopted by studies that do not have access to a wide range of potential risk factors may also be prone to residual confounding.
To address some of these limitations, we used a ‘doubly robust estimator’ to estimate the association between endometriosis and adverse pregnancy outcomes. This approach offers an opportunity to achieve unbiased inference while accounting for selection effects by combining inverse probability weighting and regression adjustment and allows for a causal interpretation of the results [24, 25]. Findings from this approach can be directly interpreted as the risk of adverse pregnancy outcomes given that women had endometriosis as compared to the counterfactual scenario in that they had no endometriosis. This causal interpretation is usually not possible from classical epidemiological approaches. This study aimed to estimate the effect (average treatment effect) of endometriosis on adverse pregnancy and perinatal outcomes using a large population-based cohort in Western Australia (WA).
Methods
Study design
We conducted a population-based, longitudinal cohort study including all women 15 to 49 years of age with a singleton pregnancy in the period of 1980–2015 in WA.
Data sources and study population
We obtained maternal, infant and birth information from the Midwives Notification System, a validated database [26] that includes > 99% of births in WA of at least 20 weeks’ gestation or birthweight of 400 g or more if the gestational age was unknown [27]. We sourced hospitalization records from the Hospital Morbidity Data Collection, which includes information on all hospitalizations from public, private and day procedure facilities in the state with International Classification of Diseases (ICD-9/10th revision-Australian Modification) coded diagnoses [28]. Data sources have been described in detail elsewhere [29]. Data were probabilistically linked using best practice protocols through the WA Data Linkage Branch [30].
From a total of 487,297 women (964,015 births) during the study period, we sequentially excluded multiple gestations, stillbirths, and pregnancies with missing information for gestational age, outcomes, maternal age, and socioeconomic status (SES). This resulted in 468,778 eligible women who contributed to 912,747 singleton pregnancies included in the analytic cohort (Fig. S1).
Exposure assessment
We identified all women with a principal or additional diagnosis of endometriosis from the hospital separation data using the International Classification of Diseases (ICD)-AM (Australian Modification) diagnostic codes consistent with ICD-9: 617.0–617.9; ICD-10: N80.0-N80.9 and Australian Classification of Health Interventions (ACHI) for endometriosis-related procedures (codes are shown in Table S5). Women were categorized as having endometriosis if they had hospital admission or surgical procedure coded as a diagnosis of endometriosis. We included women diagnosed before and after pregnancy in the primary analysis because recent studies documented a diagnosis delay of 8–12 years [4,5,6]. This approach has been adopted by other recent studies [15, 19].
Outcomes
The outcomes of interest were ascertained from the Midwives’ Notifications System and hospital separation data in the state, with the diagnostic codes consistent with preeclampsia (ICD-9/ICD-9-CM: 642.4, 642.5, 642.7, ICD-10-AM: O14, O11) and placenta previa, with or without haemorrhage (ICD-9/ICD-9-CM: 641.0–641.1, ICD-10-AM: O44.-). The onset of preeclampsia at the gestational age between 20 and 34 weeks and after 34 weeks of gestation was classified as early or late-onset preeclampsia, respectively. Preterm birth was defined as birth before 37 completed weeks of gestation, categorized into moderate preterm birth (gestational week 32–36) and very preterm birth (prior to 32 gestational weeks). Further, we also categorized preterm birth into spontaneous (due to spontaneous onset of labour) and medically indicated (due to elective caesarean section, or induction of labour). The details of ICD codes used to define variables for analysis are presented in Table S5.
Covariates
Information on potential confounding factors including the calendar year of birth of the child (categorical variable), maternal age group (15–24, 25–29, 30–34, 35–39, 40–49 years), parity (0, 1, 2, ≥ 3), smoking during pregnancy (Yes vs No), race/ethnicity (Caucasian versus non-Caucasian), and socioeconomic status (SES) was obtained from the databases. SES was measured using Socio-Economic Indexes for Areas (SEIFA). Specifically, we used the Index of Relative Socio-economic Disadvantage level at the time of birth of the child. These scores were obtained from the Australian Bureau of Statistics [31] and categorized into quintiles.
To assess the potential impact of Medically Assisted Reproduction (MAR) or infertility treatment on perinatal outcomes, we identified pregnancies with MAR procedure (using ACHI) or the following ICD diagnostic codes; ICD-9: 628.0-628.9, V26.1-V26.9, and ICD-10-AM: N97.0-97.9; Z31.1-Z31.9. These codes cover assisted reproductive technology (ART) techniques, intrauterine insemination, and ovulation induction and might include some spontaneously conceived pregnancies in couples with fertility issues [32].
Statistical analysis
We first estimated the unadjusted relative risks (RRs) with 95% confidence intervals (CI) using generalized linear models (GLM) fitted using a Poisson distribution and a log link function. Next, we estimated the causal effect (average treatment effect) of endometriosis on adverse pregnancy outcomes using the potential outcome approach, which allows for the estimation of causal effects in large observational data [33]. This was done by combining the inverse probability of treatment weighting (IPTW) and the outcome regression model in a doubly robust estimation [34]. IPTW weights each person by the inverse of their propensity score. The doubly robust estimation allows us to estimate the unbiased average causal effect when either the outcome regression model (traditional way of obtaining treatment effect) or the propensity score model (treatment selection model) is correctly specified [24, 25]. We estimated the adjusted RRs with 95% CI for each outcome derived using modified Poisson regression with a robust error variance to account for the effects of repeated pregnancies per mother [35]. We fitted the exposure model with maternal age, birth year, SES, ethnicity/race, and MAR treatment, and the outcome model with maternal age, birth year, SES, ethnicity/race and parity. As preeclampsia and placenta previa may influence the risk of preterm birth, gestational age (< 32, 32–36, > 37 weeks of gestation) was also included in the exposure model as a covariate for all outcomes included except for preterm birth. To examine the influence of MAR on the association between endometriosis and adverse pregnancy outcomes, we included a sub-analysis stratified by MAR status (Table 3). To check the covariate balance after propensity score matching using IPTW, we performed diagnostics including standardized differences and % of bias in means of all covariates (Fig. S2, Table S7). The standardized differences describe between-group differences in units of standard deviation. The confounders included in the treatment weighting were decided based on prior knowledge as well as consideration of Directed Acyclic Graphs (DAGs) (Fig. S3).
Missing data
For the main results, we conducted a complete case analysis as the proportion of missing data was small (< 3%, range 0.6% for gestational age to 1.8% for SES).
Sensitivity analysis
To check the robustness of our findings, we conducted several sensitivity analyses. Firstly, to ascertain the sensitivity of our result to higher-order parity, we restricted the analysis to primiparous women. Secondly, to limit the possibility of misclassification bias, we conducted an analysis restricted to women (i) with a principal diagnosis of endometriosis, a diagnosis established to be chiefly responsible for occasioning an episode [28]; (ii) with any diagnosis of endometriosis prior to the birth of the child to ensure endometriosis was present during pregnancy; (iii) with endometriosis diagnosis before delivery and up to five years after delivery; and (iv) considering endometriosis diagnosis at more than one-time point during five years look-up period. Thirdly, to explore the potential influence of maternal smoking during pregnancy, which was routinely collected in the Midwifery notification from 1997 onwards, we conducted a separate analysis adjusting for smoking. Next, we compared the effect of endometriosis on preterm birth (very preterm vs moderate). Fifth, we undertake a causal mediation analysis based on the counterfactual framework using a parametric regression approach [36] to estimate the natural direct effect of endometriosis compared with the natural indirect effect through MAR. Finally, to assess the extent of unmeasured confounding, we calculated E-values, which represent the minimum strength of association on the risk ratio scale, that any unmeasured confounder would need to have with both endometriosis and each outcome to fully explain away the observed association, conditional on the measured covariates. [37]
All analyses were performed using Stata version 16.1 (Stata Corporation, College Station, Texas, USA).
Results
Cohort characteristics
In total, we included 912,747 eligible singleton births with a gestational age of 20–44 weeks from women (n = 468,778) aged 15–49 years in the study period between 1980 and 2015 in WA. In these pregnancies 8874 women (1.9%) had a diagnosis of endometriosis, corresponding to 19,476 pregnancies (2.1%). Women with endometriosis were on average of advanced age at the time of birth (> 35 years), Caucasian, and had a higher proportion of medically assisted reproduction compared to women without endometriosis. Socio-economic status, parity, and ethnicity were similar among exposed and non-exposed groups (Table 1).
The prevalence of pregnancy complications was higher among pregnancies of women with endometriosis compared to women without endometriosis (preeclampsia, 7.5% vs. 6.1%; preterm birth, 10.2% vs. 7.0%; placenta previa, 1.9% vs. 1.0%; Table 2).
Using doubly robust estimation, pregnancies in women with a diagnosis of endometriosis were associated with a higher risk of preeclampsia (RR 1.18, 95% CI 1.11–1.26), placenta previa (RR 1.59, 95% CI 1.42–1.79), and preterm birth (RR 1.45, 95% CI 1.37–1.54). This risk associated with endometriosis was higher for medically indicated preterm birth (RR 1.74, 95% CI 1.58–1.93) compared to spontaneous preterm birth (RR 1.40, 95% CI 1.27–1.56) (Table 2). Furthermore, endometriosis was associated with both moderate and very preterm birth with a stronger association observed for very preterm birth (Table S3). In a stratified analysis based on MAR status, the higher risk of placenta previa and preterm birth persisted regardless of conception mode with the strongest effect estimates for the non-MAR group (RR 1.77, 95% CI 1.50–2.08 for placenta previa and RR 1.67, 95% CI 1.55–1.80 for preterm birth) and slightly attenuated effect estimates among the MAR group. However, for preeclampsia, the observed association disappeared when stratified by MAR status (Table 3).
Results of the sensitivity analyses
The results restricted to nulliparous women were consistent with the main finding with a slight attenuation (Table S2; Model 2). Findings from the analyses restricted to a subset of the study population with different exposure definitions were very similar to those reported in the main analyses (Table S2; Model 3–6). Analyses restricted to pregnancies from women with endometriosis diagnosed before delivery also resulted in slightly higher risk estimates for all adverse pregnancy outcomes evaluated (Table S2; Model 4). Additionally, the pattern of the association between endometriosis and adverse pregnancy outcomes was similar when further adjusted to smoking status (Table S2; Model 7). Our mediation analyses suggest that the percentage of endometriosis effect on preterm birth and placenta previa that was mediated through MAR was 8% and 3% respectively. (Table S4). The E-values for the observed RRs varied from 1.64 to 2.87 for these three adverse pregnancy outcomes (Table S6).
Discussion
Principal findings
To our knowledge, this is the first population-based retrospective cohort study to examine the association between endometriosis with adverse pregnancy outcomes using the potential outcome framework. Using a large (~ 1 million births) cohort in WA, we observed a higher risk of preeclampsia, placenta previa, and preterm birth among pregnancies in women with endometriosis as compared to women without endometriosis. The associations persisted after stratification for conception mode (MAR or natural conception, non-MAR), with an elevated risk among the non-MAR group meaning the risks observed were attenuated among pregnancies conceived by MAR. The risk for adverse pregnancy outcomes was higher (approximately 24%, 56%, and 85% of increased risk of preeclampsia, preterm birth, and placenta previa, respectively) when we restricted our sample to women with endometriosis as the principal diagnosis code, suggesting probably more severe disease. These observed associations were not mediated through MAR.
Strengths and limitations
Our cohort was based on longitudinally linked, highly reliable sources of population-based perinatal information ascertained from hospital separations and midwives’ notifications. We also included sensitivity analysis to check the robustness of our result. Our cohort is less prone to exposure misclassification bias since the hospital morbidity data collection (source data for our exposure) contains records for all hospital separations of admitted patients from all public and private hospitals in WA. Furthermore, our study restricted the analysis to singleton pregnancies, which improved generalizability to other similar cohorts.
We only had information on the diagnosis of endometriosis for women who have been hospitalized during the study period. Such data may likely represent more severe stages of endometriosis. The clinical routines and obstetric care have changed through the years and the diagnosis and awareness regarding endometriosis have evolved. However, to minimize this bias our model included the birth year of the child as a covariate. In our main analysis, we included all women with any diagnosis of endometriosis (principal and additional diagnosis). This could have introduced non-differential misclassification bias (i.e., independent of the outcome) and, therefore, will potentially bias the results towards the null. To limit this possible misclassification, we included a sensitivity analysis restricted to an exposure defined as a principal diagnosis of endometriosis, which indicated higher risk estimates as compared to the main result. We opted to include women with a diagnosis of endometriosis before and after pregnancy to account for the diagnostic delay [4, 5]. This could induce similar misclassification bias and attenuation of the association. Indeed, our sensitivity analysis restricted to a diagnosis of endometriosis before delivery consistently suggested higher effect estimates as compared to the main analysis. Though the validity of the diagnosis of endometriosis in the hospital separation database remains unknown, previous analysis of the same database suggested that endometriosis is reliably recorded in the hospital separation data [38]. Moreover, while the use of ICD and procedure codes ensures that those classified as having endometriosis are likely true cases, there is the possibility that the comparison group may have undiagnosed endometriosis. Our cohort had a relatively small number of events for stillbirths to be considered as an outcome. We, therefore, excluded pregnancies resulting in stillbirths from our analysis. This may have introduced a livebirth bias in the association between endometriosis and adverse pregnancy outcomes. However, a previous simulation study indicated that the magnitude of this bias is small [39]. Despite the potential susceptibility of doubly robust estimation to the limitations of misspecification bias, our study employs directed acyclic graphs (DAGs) in the selection of variables for the exposure and outcome models which helps to address the limitation and strengthens the robustness of our results.
Interpretation
Our study observed a modest association between endometriosis and preeclampsia, which is consistent with previous studies [12, 15, 20, 21]. However, other studies have reported a lower risk of preeclampsia [13, 17] with some suggesting no association [14]. These controversial results may be related to sample size, heterogeneity in exposure or outcome definition, selection bias, not taking MAR into account, diagnostic methods, and disease heterogeneity.
The attenuated risk in women who received MAR treatment is supported by other studies that did not find an association or a reduced association between endometriosis and preeclampsia or hypertension in pregnancy in women with an endometriosis and MAR procedure. [40, 41] Our study also found an increased risk of placenta previa in pregnancies among women with endometriosis, which is consistent with other research [20]. It has been suggested that the association may be confounded by the increased use of MAR in women with endometriosis. In our study, the association persisted even after stratification by MAR status, with a stronger association observed in non-MAR women which is consistent with other studies [40]. In our study, for women using MAR, the precision of the effect estimates was reduced likely because of the small sample size. For preterm birth, we observed higher risk estimates for very preterm deliveries compared to moderate preterm deliveries, and an association between endometriosis and both spontaneous and medically indicated preterm birth, with a stronger association for medically indicated preterm birth. This could imply that pregnancies from women with endometriosis are more likely to be induced or delivered through a caesarean section before gestational week 37. This finding is consistent with previous research and the association seems independent of MAR. [20, 41, 42] A smaller protective effect was also observed in a Canadian study [12].
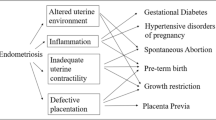
Endometriosis may be associated with adverse pregnancy outcomes through various mechanisms, including effects on the uterine environment, progesterone signalling, and the remodelling of the spiral artery [18, 43, 44]. These factors may play a role in the association with preeclampsia, preterm birth and intrauterine growth restriction [45,46,47]. Endometriotic lesions in the uterus may also reduce uterine contractility and cause abnormal implantation, leading to placenta previa [48]. In our sub-analysis of the timing of preeclampsia, we found an elevated risk for early onset compared to late-onset preeclampsia, which can be accounted for inadequate and incomplete trophoblast invasion of maternal spiral arteries [43]. MAR treatment itself has shown to be a risk factor for adverse pregnancy outcomes, with mixed results for women with endometriosis [47]. In MAR treatment, the effects caused by endometriosis such as inflammatory processes and regulatory disbalances are suppressed offering a better pregnancy environment and could explain the attenuation in the risks seen in our study in the MAR pregnancies group [49]. Women conceiving following MAR might also have support from better obstetric care and closer screening for adverse outcomes.
In this study, a stronger association between endometriosis and adverse pregnancy outcomes was observed, but residual and/or unmeasured confounding could not be completely ruled out. Nevertheless, the E-values for the observed RRs (ranging from 1.64 to 2.87) indicated that substantial confounding would need to explain away these associations (Table S6). Systematic reviews that examined the risk factors for endometriosis, for example, reported RRs ranging from 1.63 for smoking to 1.87 for overweight—lower than that of the E-values. [50, 51] In general, findings from our sensitivity analyses were remarkably similar to those reported for the main analysis and collectively support the hypothesis that endometriosis is associated with adverse pregnancy outcomes independent of MAR. Therefore, knowledge of a patient's endometriosis history may inform targeted prenatal care and reduce unfavourable pregnancy outcomes. Future studies would benefit from elucidating the potential mechanism that might explain how endometriosis affects implantation, placentation, and fetal growth and identifying potential interventions to decrease the risk of adverse perinatal outcomes.
Conclusion
In conclusion, regardless of the use of medically assisted reproduction, endometriosis is associated with an increased risk of preeclampsia, placenta previa, and preterm birth. These findings offer new insight into the causal association between endometriosis on adverse pregnancy outcomes, taking MAR into account. This may help to enhance future obstetric care among this population.
Data availability
No additional data are available.
References
Macer ML, Taylor HS (2012) Endometriosis and infertility: a review of the pathogenesis and treatment of endometriosis-associated infertility. Obstet Gynecol Clin 39(4):535–549
Farquhar C (2007) Endometriosis. BMJ 334(7587):249–253
Nnoaham KE, Hummelshoj L, Webster P, d’Hooghe T, de Cicco NF, de Cicco NC et al (2011) Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril 96(2):366–373
Ghai V, Jan H, Shakir F, Haines P, Kent A (2020) Diagnostic delay for superficial and deep endometriosis in the United Kingdom. J Obstet Gynaecol 40(1):83–89
Hudelist G, Fritzer N, Thomas A, Niehues C, Oppelt P, Haas D et al (2012) Diagnostic delay for endometriosis in Austria and Germany: causes and possible consequences. Hum Reprod 27(12):3412–3416
Armour M, Sinclair J, Ng CHM, Hyman MS, Lawson K, Smith CA et al (2020) Endometriosis and chronic pelvic pain have similar impact on women, but time to diagnosis is decreasing: an Australian survey. Sci Rep 10(1):16253
Carter JE (1994) Combined hysteroscopic and laparoscopic findings in patients with chronic pelvic pain. J Am Assoc Gynecol Laparosc 2(1):43–47
Shafrir AL, Farland L, Shah D, Harris H, Kvaskoff M, Zondervan K et al (2018) Risk for and consequences of endometriosis: a critical epidemiologic review. Best Pract Res Clin Obstet Gynaecol 51:1–15
Health AIo (2019) Welfare endometriosis in Australia: prevalence and hospitalisations. AIHW, Canberra
Prescott J, Farland LV, Tobias DK, Gaskins AJ, Spiegelman D, Chavarro JE et al (2016) A prospective cohort study of endometriosis and subsequent risk of infertility. Hum Reprod (Oxford, England) 31(7):1475–1482
Sorrentino F, Falagario M, D’Alterio M, Carugno J, Nappi L (2021) Endometriosis and adverse pregnancy outcome. Minerva Obstet Gynecol. https://doi.org/10.23736/S2724-606X.20.04718-8
Velez MP, Bougie O, Bahta L, Pudwell J, Griffiths R, Li W et al (2022) Mode of conception in patients with endometriosis and adverse pregnancy outcomes: a population-based cohort study. Fertil Steril. https://doi.org/10.1016/j.fertnstert.2022.09.015
Stephansson O, Kieler H, Granath F, Falconer H (2009) Endometriosis, assisted reproduction technology, and risk of adverse pregnancy outcome. Hum Reprod 24(9):2341–2347
Hadfield RM, Lain SJ, Raynes-Greenow CH, Morris JM, Roberts CL (2009) Is there an association between endometriosis and the risk of pre-eclampsia? A population based study. Hum Reprod 24(9):2348–2352
Glavind MT, Forman A, Arendt LH, Nielsen K, Henriksen TB (2017) Endometriosis and pregnancy complications: a Danish cohort study. Fertil Steril 107(1):160–166
Farland LV, Prescott J, Sasamoto N, Tobias DK, Gaskins AJ, Stuart JJ et al (2019) Endometriosis and risk of adverse pregnancy outcomes. Obstet Gynecol 134(3):527
Brosens IA, De Sutter P, Hamerlynck T, Imeraj L, Yao Z, Cloke B et al (2007) Endometriosis is associated with a decreased risk of pre-eclampsia. Hum Reprod 22(6):1725–1729
Brosens I, Brosens JJ, Fusi L, Al-Sabbagh M, Kuroda K, Benagiano G (2012) Risks of adverse pregnancy outcome in endometriosis. Fertil Steril 98(1):30–35
Breintoft K, Arendt LH, Uldbjerg N, Glavind MT, Forman A, Henriksen TB (2022) Endometriosis and preterm birth: a Danish cohort study. Acta Obstet Gynecol Scand 101(4):417–423
Breintoft K, Pinnerup R, Henriksen TB, Rytter D, Uldbjerg N, Forman A et al (2021) Endometriosis and risk of adverse pregnancy outcome: a systematic review and meta-analysis. J Clin Med 10(4):667
Farland LV, Stern JE, Liu C-l, Cabral HJ, Coddington CC III, Diop H et al (2022) Pregnancy outcomes among women with endometriosis and fibroids: registry linkage study in Massachusetts. Am J Obstet Gynecol. https://doi.org/10.1016/j.ajog.2021.12.268
Farland LV, Davidson S, Sasamoto N, Horne AW, Missmer SA (2020) Adverse pregnancy outcomes in endometriosis-myths and realities. Curr Obstet Gynecol Rep 9(1):27–35
Herbert DL, Lucke JC, Dobson AJ (2009) Infertility, medical advice and treatment with fertility hormones and/or in vitro fertilisation: a population perspective from the Australian longitudinal study on women;s Health. Aust N Z J Public Health 33(4):358–364
Austin PC (2010) The performance of different propensity-score methods for estimating differences in proportions (risk differences or absolute risk reductions) in observational studies. Stat Med 29(20):2137–2148
Funk MJ, Westreich D, Wiesen C, Stürmer T, Brookhart MA, Davidian M (2011) Doubly robust estimation of causal effects. Am J Epidemiol 173(7):761–767
Downey F (2007) A validation study of the Western Australian Midwives’ notification system. 2005 data. Department of Health, Western Australia, Perth
Department of Health Western Australia. Midwives Notification System. [Internet] 2017. Available from: http://ww2.health.wa.gov.au/Articles/J_M/Midwives-Notification-System. Accessed 28 Nov 2017
Australia DOHW (2004) Hospital morbdiity data system reference manual July 2004. Department of HealthWestern Australia, Health Data Collections Branch, Health Information Centre, Perth
Gebremedhin AT, Regan AK, Ball S, Betrán AP, Foo D, Gissler M, Håberg SE, Malacova E, Marinovich ML, Pereira G (2020) Interpregnancy interval and hypertensive disorders of pregnancy: a population-based cohort study. Paediatr Perinatal Epidemiol 35:404–414. https://doi.org/10.1111/ppe.12668
Holman CDAJ, Bass AJ, Rouse IL, Hobbs MS (1999) Population-based linkage of health records in Western Australia: development of a health services research linked database. Aust N Z J Public Health 23(5):453–459
Australian Bureau of Statistics. Socio-Economic Indexes for Areas. 2017 23 September 2013. Available from: http://www.abs.gov.au/websitedbs/censushome.nsf/home/seifa. Accessed 28 Nov 2017
Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, De Mouzon J, Sokol R et al (2017) The international glossary on infertility and fertility care, 2017. Hum Reprod 32(9):1786–1801
Rubin DB (2005) Causal inference using potential outcomes. J Am Stat Assoc 100(469):322–331
Bang H, Robins JM (2005) Doubly robust estimation in missing data and causal inference models. Biometrics 61(4):962–973
Zou G (2004) A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 159(7):702–706
Samoilenko M, Lefebvre G (2021) Parametric-regression–based causal mediation analysis of binary outcomes and binary mediators: moving beyond the rareness or commonness of the outcome. Am J Epidemiol 190(9):1846–1858
VanderWeele TJ, Ding P (2017) Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med 167(4):268–274
Spilsbury K, Semmens J, Hammond I, Bolck A (2006) Persistent high rates of hysterectomy in Western Australia: a population-based study of 83000 procedures over 23 years. BJOG 113(7):804–809
Liew Z, Olsen J, Cui X, Ritz B, Arah OA (2015) Bias from conditioning on live birth in pregnancy cohorts: an illustration based on neurodevelopment in children after prenatal exposure to organic pollutants. Int J Epidemiol 44(1):345–354
Epelboin S, Labrosse J, Fauque P, Levy R, Gervoise-Boyer MJ, Devaux A et al (2021) Endometriosis and assisted reproductive techniques independently related to mother-child morbidities: a French longitudinal national study. Reprod Biomed Online 42(3):627–633
Ibiebele I, Nippita T, Baber R, Torvaldsen S (2022) Pregnancy outcomes in women with endometriosis and/or ART use: a population-based cohort study. Hum Reprod. https://doi.org/10.1093/humrep/deac186
Pérez-López F, Villagrasa-Boli P, Muñoz-Olarte M, Morera-Grau Á, Cruz-Andrés P, Hernandez A (2018) Health outcomes and systematic analyses (HOUSSAY) project association between endometriosis and preterm birth in women with spontaneous conception or using assisted reproductive technology: a systematic review and meta-analysis of cohort studies. Reprod Sci 25:311–319
Fiorentino G, Cimadomo D, Innocenti F, Soscia D, Vaiarelli A, Ubaldi FM et al (2022) Biomechanical forces and signals operating in the ovary during folliculogenesis and their dysregulation: implications for fertility. Hum Reprod Update. https://doi.org/10.1093/humupd/dmac031
Joshi NR, Miyadahira EH, Afshar Y, Jeong J-W, Young SL, Lessey BA et al (2017) Progesterone resistance in endometriosis is modulated by the altered expression of microRNA-29c and FKBP4. J Clin Endocrinol Metab 102(1):141–149
Kunz G, Beil D, Huppert P, Leyendecker G (2000) Structural abnormalities of the uterine wall in women with endometriosis and infertility visualized by vaginal sonography and magnetic resonance imaging. Hum Reprod 15(1):76–82
Brosens I, Pijnenborg R, Benagiano G (2013) Defective myometrial spiral artery remodelling as a cause of major obstetrical syndromes in endometriosis and adenomyosis. Placenta 34(2):100–105
Vigano P, Corti L, Berlanda N (2015) Beyond infertility: obstetrical and postpartum complications associated with endometriosis and adenomyosis. Fertil Steril 104(4):802–812
Leone Roberti Maggiore U, Ferrero S, Mangili G, Bergamini A, Inversetti A, Giorgione V et al (2016) A systematic review on endometriosis during pregnancy: diagnosis, misdiagnosis, complications and outcomes. Hum Reprod Update 22(1):70–103
Pirtea P, de Ziegler D, Ayoubi JM (2021) Effects of endometriosis on assisted reproductive technology: gone with the wind. Fertil Steril 115(2):321–322
Bravi F, Parazzini F, Cipriani S, Chiaffarino F, Ricci E, Chiantera V et al (2014) Tobacco smoking and risk of endometriosis: a systematic review and meta-analysis. BMJ Open 4(12):e006325
Jenabi E, Khazaei S, Veisani Y (2019) The association between body mass index and the risk of endometriosis: a meta-analysis. J Endometr Pelvic Pain Disord 11(2):55–61
Acknowledgements
The authors would like to thank the Data Linkage Branch (Department of Health Western Australia) as well as the Data custodian for the Midwives Notification System and Hospital Morbidity Data Collection for providing data for this project.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. GP was supported with funding from the National Health and Medical Research Council Project and Investigator Grants #1099655 and #1173991, and the Research Council of Norway through its Centres of Excellence funding scheme #262700. GAT was supported with funding from the National Health and Medical Research Council Investigator Grant #1195716. The funders had no role in the analysis, interpretations of the results, writing of the reports, and the decision to submit the paper for possible publication.
Author information
Authors and Affiliations
Contributions
ATG: project development, data management and analysis, initial data interpretation, wrote the first draft of the manuscript. VRM, BD, GAT, and GP: substantial contributions to the statistical analyses, data interpretation, and critical revisions of the subsequent drafts of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no potential conflicts of interest to disclose.
Ethical approval
This study was conducted in accordance with the principles of the Declaration of Helsinki. This research was approved by the Human Research Ethics Committee (HREC approval 2016/51) from the Department of Health, WA. The Ethics Committee approval was accepted on 14 September 2016.
Consent to participate
Consent for the study was obtained from the data custodians. As the study was based on routinely collected de-identified linked administrative data, individual consent from the participants was not obtained.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gebremedhin, A.T., Mitter, V.R., Duko, B. et al. Associations between endometriosis and adverse pregnancy and perinatal outcomes: a population-based cohort study. Arch Gynecol Obstet 309, 1323–1331 (2024). https://doi.org/10.1007/s00404-023-07002-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-023-07002-y