Abstract
Purpose
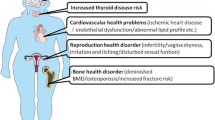
Premature ovarian insufficiency (POI) is a clinical syndrome defined by loss of ovarian activity before the age of 40 years. However, the etiology of approximately 90% patients remains unknown. Diminished ovarian reserve (DOR) and poor ovarian response (POR) are related to POI in clinic. The main purpose of this review was to evaluate the roles of microRNAs (miRNAs) in the pathogenesis and therapeutic potential for POI, DOR and POR.
Methods
A literature search was conducted using six databases (PubMed, EMBASE, Web of Science, Cochrane Library, CNKI and Wangfang Data) to obtain relevant studies.
Results
This review enlightens expression profiles and functional studies of miRNAs in ovarian insufficiency in animal models and humans. Functional studies emphasized the role of miRNAs in steroidogenesis, granulosa cell proliferation/apoptosis, autophagy and follicular development by regulating target genes in specific pathways, such as the PI3K/AKT/mTOR, TGFβ, MAPK and Hippo pathways. Differentially expressed circulating miRNAs provided novel biomarkers for diagnosis and prediction, such as miR-22-3p and miR-21. Moreover, exosomes derived from stem cells restored ovarian function through miRNAs in chemotherapy-induced POI models.
Conclusion
Differential miRNA expression profiles in patients and animal models uncovered the underlying mechanisms and biomarkers of ovarian insufficiency. Exosomal miRNAs can restore ovarian function against chemotherapy-induced POI, which needs further investigation to develop novel preventive and therapeutic strategies in clinical practice.

Similar content being viewed by others
Data availability
The data generated or analyzed during the current study are available from the corresponding author on reasonable request.
References
European Society for Human, R., Embryology Guideline Group on, P. O. I., Webber L et al (2016) Eshre guideline: management of women with premature ovarian insufficiency. Hum Reprod 31(5):926–937. https://doi.org/10.1093/humrep/dew027
Golezar S, Ramezani Tehrani F, Khazaei S et al (2019) The global prevalence of primary ovarian insufficiency and early menopause: a meta-analysis. Climacteric 22(4):403–411. https://doi.org/10.1080/13697137.2019.1574738
Nelson LM, Covington SN, Rebar RW (2005) An update: spontaneous premature ovarian failure is not an early menopause. Fertil Steril 83(5):1327–1332. https://doi.org/10.1016/j.fertnstert.2004.11.059
Welt CK (2008) Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf) 68(4):499–509. https://doi.org/10.1111/j.1365-2265.2007.03073.x
Pastore LM, Christianson MS, Stelling J et al (2018) Reproductive ovarian testing and the alphabet soup of diagnoses: dor, poi, pof, por, and for. J Assist Reprod Genet 35(1):17–23. https://doi.org/10.1007/s10815-017-1058-4
Sharara FI, Scott RT Jr, Seifer DB (1998) The detection of diminished ovarian reserve in infertile women. Am J Obstet Gynecol 179(3 Pt 1):804–812. https://doi.org/10.1016/s0002-9378(98)70087-0
Ferraretti AP, La Marca A, Fauser BC et al (2011) Eshre consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the bologna criteria. Hum Reprod 26(7):1616–1624. https://doi.org/10.1093/humrep/der092
Szmidt NA, Bhattacharya S, Maheshwari A (2016) Does poor ovarian response to gonadotrophins predict early menopause? A retrospective cohort study with minimum of 10-year follow-up. Hum Fertil (Camb) 19(3):212–219. https://doi.org/10.1080/14647273.2016.1221149
Guo Y, Sun J, Lai D (2017) Role of micrornas in premature ovarian insufficiency. Reprod Biol Endocrinol 15(1):38. https://doi.org/10.1186/s12958-017-0256-3
Pankiewicz K, Laudanski P, Issat T (2021) The role of noncoding rna in the pathophysiology and treatment of premature ovarian insufficiency. Int J Mol Sci. https://doi.org/10.3390/ijms22179336
Ambros V (2004) The functions of animal micrornas. Nature 431(7006):350–355. https://doi.org/10.1038/nature02871
Ro S, Song R, Park C et al (2007) Cloning and expression profiling of small rnas expressed in the mouse ovary. RNA 13(12):2366–2380. https://doi.org/10.1261/rna.754207
Kuang H, Han D, Xie J et al (2014) Profiling of differentially expressed micrornas in premature ovarian failure in an animal model. Gynecol Endocrinol 30(1):57–61. https://doi.org/10.3109/09513590.2013.850659
Ai A, Xiong Y, Wu B et al (2018) Induction of mir-15a expression by tripterygium glycosides caused premature ovarian failure by suppressing the hippo-yap/taz signaling effector lats1. Gene 678:155–163. https://doi.org/10.1016/j.gene.2018.08.018
Liu T, Liu Y, Huang Y et al (2019) Mir-15b induces premature ovarian failure in mice via inhibition of α-klotho expression in ovarian granulosa cells. Free Radic Biol Med 141:383–392. https://doi.org/10.1016/j.freeradbiomed.2019.07.010
Zhao H, Gu W, Pan W et al (2021) mir-483-5p aggravates cisplatin-induced premature ovarian insufficiency in rats by targeting fkbp4. Nan Fang Yi Ke Da Xue Xue Bao 41(6):801–810. https://doi.org/10.12122/j.issn.1673-4254.2021.06.01
Zhang X, Zhang R, Hao J et al (2022) Mirna-122-5p in poi ovarian-derived exosomes promotes granulosa cell apoptosis by regulating bcl9. Cancer Med 11(12):2414–2426. https://doi.org/10.1002/cam4.4615
Liu C, Li Q, Yang Y (2019) Effects of the modified bazhen decoction in the treatment of premature ovarian failure in rats. Ann Clin Lab Sci 49(1):16–22
Yang X, Zhou Y, Peng S et al (2012) Differentially expressed plasma micrornas in premature ovarian failure patients and the potential regulatory function of mir-23a in granulosa cell apoptosis. Reproduction 144(2):235–244. https://doi.org/10.1530/REP-11-0371
Kim YJ, Ku SY, Kim YY et al (2013) Micrornas transfected into granulosa cells may regulate oocyte meiotic competence during in vitro maturation of mouse follicles. Hum Reprod 28(11):3050–3061. https://doi.org/10.1093/humrep/det338
Tao H, Wang L, Zhou J et al (2015) The transcription factor ccaat/enhancer binding protein β (c/ebpβ) and mir-27a regulate the expression of porcine dickkopf2 (dkk2). Sci Rep 5:17972. https://doi.org/10.1038/srep17972
Tao H, Xiong Q, Ji Z et al (2019) Nfat5 is regulated by p53/mir-27a signal axis and promotes mouse ovarian granulosa cells proliferation. Int J Biol Sci 15(2):287–297. https://doi.org/10.7150/ijbs.29273
Liu T, Lin J, Chen C et al (2021) Microrna-146b-5p overexpression attenuates premature ovarian failure in mice by inhibiting the dab2ip/ask1/p38-mapk pathway and gammah2a.X phosphorylation. Cell Prolif 54(1):e12954. https://doi.org/10.1111/cpr.12954
Zhang M, Zhang Q, Hu Y et al (2017) Mir-181a increases foxo1 acetylation and promotes granulosa cell apoptosis via sirt1 downregulation. Cell Death Dis 8(10):e3088. https://doi.org/10.1038/cddis.2017.467
Zhang C, Shen J, Kong S et al (2019) Microrna-181a promotes follicular granulosa cell apoptosis via sphingosine-1-phosphate receptor 1 expression downregulation†. Biol Reprod 101(5):975–985. https://doi.org/10.1093/biolre/ioz135
Zhang Q, Sun H, Jiang Y et al (2013) Microrna-181a suppresses mouse granulosa cell proliferation by targeting activin receptor iia. PLoS ONE 8(3):e59667. https://doi.org/10.1371/journal.pone.0059667
Zhang JQ, Gao BW, Guo HX et al (2020) Mir-181a promotes porcine granulosa cell apoptosis by targeting tgfbr1 via the activin signaling pathway. Mol Cell Endocrinol 499:110603. https://doi.org/10.1016/j.mce.2019.110603
Dang Y, Zhao S, Qin Y et al (2015) Microrna-22-3p is down-regulated in the plasma of han chinese patients with premature ovarian failure. Fertil Steril 103(3):802-807.e801. https://doi.org/10.1016/j.fertnstert.2014.12.106
Sirotkin AV, Laukova M, Ovcharenko D et al (2010) Identification of micrornas controlling human ovarian cell proliferation and apoptosis. J Cell Physiol 223(1):49–56. https://doi.org/10.1002/jcp.21999
Xiong F, Hu L, Zhang Y et al (2016) Mir-22 inhibits mouse ovarian granulosa cell apoptosis by targeting sirt1. Biol Open 5(3):367–371. https://doi.org/10.1242/bio.016907
Zhang X, Dang Y, Liu R et al (2020) Microrna-127-5p impairs function of granulosa cells via hmgb2 gene in premature ovarian insufficiency. J Cell Physiol 235(11):8826–8838. https://doi.org/10.1002/jcp.29725
Dang Y, Wang X, Hao Y et al (2018) Microrna-379-5p is associate with biochemical premature ovarian insufficiency through parp1 and xrcc6. Cell Death Dis 9(2):106. https://doi.org/10.1038/s41419-017-0163-8
Zhao G, Zhou X, Fang T et al (2014) Hyaluronic acid promotes the expression of progesterone receptor membrane component 1 via epigenetic silencing of mir-139-5p in human and rat granulosa cells. Biol Reprod 91(5):116. https://doi.org/10.1095/biolreprod.114.120295
Chen X, Xie M, Liu D et al (2015) Downregulation of microrna146a inhibits ovarian granulosa cell apoptosis by simultaneously targeting interleukin1 receptorassociated kinase and tumor necrosis factor receptorassociated factor 6. Mol Med Rep 12(4):5155–5162. https://doi.org/10.3892/mmr.2015.4036
Abd El Naby WS, Hagos TH, Hossain MM et al (2013) Expression analysis of regulatory micrornas in bovine cumulus oocyte complex and preimplantation embryos. Zygote 21(1):31–51. https://doi.org/10.1017/S0967199411000566
Nie M, Yu S, Peng S et al (2015) Mir-23a and mir-27a promote human granulosa cell apoptosis by targeting smad5. Biol Reprod 93(4):98. https://doi.org/10.1095/biolreprod.115.130690
Luo H, Han Y, Liu J et al (2019) Identification of micrornas in granulosa cells from patients with different levels of ovarian reserve function and the potential regulatory function of mir-23a in granulosa cell apoptosis. Gene 686:250–260. https://doi.org/10.1016/j.gene.2018.11.025
Assou S, Al-edani T, Haouzi D et al (2013) Micrornas: new candidates for the regulation of the human cumulus-oocyte complex. Hum Reprod 28(11):3038–3049. https://doi.org/10.1093/humrep/det321
Li X, Xie J, Wang Q et al (2020) Mir-21 and pellino-1 expression profiling in autoimmune premature ovarian insufficiency. J Immunol Res 2020:3582648. https://doi.org/10.1155/2020/3582648
Zhang X, Chen Y, Yang M et al (2020) Mir-21-5p actions at the smad7 gene during pig ovarian granulosa cell apoptosis. Anim Reprod Sci 223:106645. https://doi.org/10.1016/j.anireprosci.2020.106645
Aldakheel FM, Abuderman AA, Alduraywish SA et al (2021) Microrna-21 inhibits ovarian granulosa cell proliferation by targeting snhg7 in premature ovarian failure with polycystic ovary syndrome. J Reprod Immunol 146:103328. https://doi.org/10.1016/j.jri.2021.103328
Fu X, He Y, Wang X et al (2017) Overexpression of mir-21 in stem cells improves ovarian structure and function in rats with chemotherapy-induced ovarian damage by targeting pdcd4 and pten to inhibit granulosa cell apoptosis. Stem Cell Res Ther 8(1):187. https://doi.org/10.1186/s13287-017-0641-z
Ma L, Tang X, Guo S et al (2020) Mirna-21-3p targeting of fgf2 suppresses autophagy of bovine ovarian granulosa cells through akt/mtor pathway. Theriogenology 157:226–237. https://doi.org/10.1016/j.theriogenology.2020.06.021
Ma L, Zheng Y, Tang X et al (2019) Mir-21-3p inhibits autophagy of bovine granulosa cells by targeting vegfa via pi3k/akt signaling. Reproduction 158(5):441–452. https://doi.org/10.1530/REP-19-0285
Bartolucci AF, Uliasz T, Peluso JJ (2020) Microrna-21 as a regulator of human cumulus cell viability and its potential influence on the developmental potential of the oocyte. Biol Reprod 103(1):94–103. https://doi.org/10.1093/biolre/ioaa058
Hilker RE, Pan B, Zhan X et al (2021) Microrna-21 enhances estradiol production by inhibiting wt1 expression in granulosa cells. J Mol Endocrinol 68(1):11–22. https://doi.org/10.1530/JME-21-0162
Donadeu FX, Mohammed BT, Ioannidis J (2017) A mirna target network putatively involved in follicular atresia. Domest Anim Endocrinol 58:76–83. https://doi.org/10.1016/j.domaniend.2016.08.002
Carletti MZ, Fiedler SD, Christenson LK (2010) Microrna 21 blocks apoptosis in mouse periovulatory granulosa cells. Biol Reprod 83(2):286–295. https://doi.org/10.1095/biolreprod.109.081448
Dehghan Z, Mohammadi-Yeganeh S, Rezaee D et al (2021) Microrna-21 is involved in oocyte maturation, blastocyst formation, and pre-implantation embryo development. Dev Biol 480:69–77. https://doi.org/10.1016/j.ydbio.2021.08.008
Woo I, Christenson LK, Gunewardena S et al (2018) Micro-rnas involved in cellular proliferation have altered expression profiles in granulosa of young women with diminished ovarian reserve. J Assist Reprod Genet 35(10):1777–1786. https://doi.org/10.1007/s10815-018-1239-9
Chen D, Zhang Z, Chen B et al (2017) Altered microrna and piwi-interacting rna profiles in cumulus cells from patients with diminished ovarian reserve. Biol Reprod 97(1):91–103. https://doi.org/10.1093/biolre/iox062
Hong L, Peng S, Li Y et al (2018) Mir-106a increases granulosa cell viability and is downregulated in women with diminished ovarian reserve. J Clin Endocrinol Metab 103(6):2157–2166. https://doi.org/10.1210/jc.2017-02344
Ford EA, Beckett EL, Roman SD et al (2020) Advances in human primordial follicle activation and premature ovarian insufficiency. Reproduction 159(1):R15–R29. https://doi.org/10.1530/REP-19-0201
Li H, Wang X, Mu H et al (2022) Mir-484 contributes to diminished ovarian reserve by regulating granulosa cell function via yap1-mediated mitochondrial function and apoptosis. Int J Biol Sci 18(3):1008–1021. https://doi.org/10.7150/ijbs.68028
Zhang K, Zhong W, Li WP et al (2017) Mir-15a-5p levels correlate with poor ovarian response in human follicular fluid. Reproduction 154(4):483–496. https://doi.org/10.1530/rep-17-0157
Karakaya C, Guzeloglu-Kayisli O, Uyar A et al (2015) Poor ovarian response in women undergoing in vitro fertilization is associated with altered microrna expression in cumulus cells. Fertil Steril 103(6):1469–1476. https://doi.org/10.1016/j.fertnstert.2015.02.035. (e1461-1463)
Chen X, Ba Y, Ma L et al (2008) Characterization of micrornas in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res 18(10):997–1006. https://doi.org/10.1038/cr.2008.282
Abu-Halima M, Becker LS, Ayesh BM et al (2021) Characterization of micro-rna in women with different ovarian reserve. Sci Rep 11(1):13351. https://doi.org/10.1038/s41598-021-92901-w
Belguith I, Dhieb D, Turki M et al (2022) Diagnostic value of mir-199a and mir-21 in the plasma of infertile women with dysregulated amh levels. Hum Fertil (Camb) 25(1):154–165. https://doi.org/10.1080/14647273.2020.1750715
Khan HL, Bhatti S, Abbas S et al (2021) Extracellular micrornas: key players to explore the outcomes of in vitro fertilization. Reprod Biol Endocrinol 19(1):72. https://doi.org/10.1186/s12958-021-00754-9
Fàbregues F, Ferreri J, Méndez M et al (2020) In vitro follicular activation and stem cell therapy as a novel treatment strategies in diminished ovarian reserve and primary ovarian insufficiency. Front Endocrinol (Lausanne) 11:617704. https://doi.org/10.3389/fendo.2020.617704
Ding C, Qian C, Hou S et al (2020) Exosomal mirna-320a is released from hamscs and regulates sirt4 to prevent reactive oxygen species generation in poi. Mol Ther Nucl Acids 21:37–50. https://doi.org/10.1016/j.omtn.2020.05.013
Sun B, Ma Y, Wang F et al (2019) Mir-644-5p carried by bone mesenchymal stem cell-derived exosomes targets regulation of p53 to inhibit ovarian granulosa cell apoptosis. Stem Cell Res Ther 10(1):360. https://doi.org/10.1186/s13287-019-1442-3
Yang M, Lin L, Sha C et al (2020) Bone marrow mesenchymal stem cell-derived exosomal mir-144-5p improves rat ovarian function after chemotherapy-induced ovarian failure by targeting pten. Lab Invest 100(3):342–352. https://doi.org/10.1038/s41374-019-0321-y
Xiao GY, Cheng CC, Chiang YS et al (2016) Exosomal mir-10a derived from amniotic fluid stem cells preserves ovarian follicles after chemotherapy. Sci Rep 6:23120. https://doi.org/10.1038/srep23120
Zhang Q, Sun J, Huang Y et al (2019) Human amniotic epithelial cell-derived exosomes restore ovarian function by transferring micrornas against apoptosis. Mol Ther Nucl Acids 16:407–418. https://doi.org/10.1016/j.omtn.2019.03.008
Ding C, Zhu L, Shen H et al (2020) Exosomal mirna-17-5p derived from human umbilical cord mesenchymal stem cells improves ovarian function in premature ovarian insufficiency by regulating sirt7. Stem Cells 38(9):1137–1148. https://doi.org/10.1002/stem.3204
Gao T, Cao Y, Hu M et al (2022) Human umbilical cord mesenchymal stem cell-derived extracellular vesicles carrying microrna-29a improves ovarian function of mice with primary ovarian insufficiency by targeting hmg-box transcription factor/wnt/β-catenin signaling. Dis Mark 2022:5045873. https://doi.org/10.1155/2022/5045873
Qu Q, Liu L, Cui Y et al (2022) Mir-126-3p containing exosomes derived from human umbilical cord mesenchymal stem cells promote angiogenesis and attenuate ovarian granulosa cell apoptosis in a preclinical rat model of premature ovarian failure. Stem Cell Res Ther 13(1):352. https://doi.org/10.1186/s13287-022-03056-y
Yang W, Zhang J, Xu B et al (2020) Hucmsc-derived exosomes mitigate the age-related retardation of fertility in female mice. Mol Ther 28(4):1200–1213. https://doi.org/10.1016/j.ymthe.2020.02.003
Du X, Li Q, Pan Z et al (2016) Androgen receptor and mirna-126* axis controls follicle-stimulating hormone receptor expression in porcine ovarian granulosa cells. Reproduction 152(2):161–169. https://doi.org/10.1530/rep-15-0517
Funding
This work was supported by the National Natural Science Foundation of China (Grants Numbers 82071598).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection and analysis were performed by JL. The first draft of the manuscript was written by JL and edited by ZS. The authors both commented on previous versions of the manuscript and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luo, J., Sun, Z. MicroRNAs in POI, DOR and POR. Arch Gynecol Obstet 308, 1419–1430 (2023). https://doi.org/10.1007/s00404-023-06922-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-023-06922-z