Abstract
Purpose
Despite the growing understanding of the carcinogenesis of pelvic high-grade serous carcinoma (HGSC) of the ovary and peritoneum and its precursor lesion serous tubal intraepithelial carcinoma (STIC), evidence-based proven recommendations on the clinical management of patients with STIC are lacking so far.
Methods
A questionnaire containing 21 questions was developed to explore the clinical experience with patients with the diagnosis of STICs and the diagnostic, surgical and histopathological approaches in Germany. Overall, 540 clinical heads of department in all German gynaecological centres were asked to participate.
Results
131 questionnaires (response rate 24.3%) were included in this survey. 45.8% of the respondents had treated one to three STIC patients during their career. 75.6% of the respondents performed opportunistic bilateral salpingectomies during other gynaecological surgeries. Most of the participants (31.3%) started with the SEE-FIM (Sectioning and Extensively Examining the FIMbria) protocol in 2014. It was requested by 39.7% centres for prophylactic salpingectomies, by 13.7% for both prophylactic and opportunistic salpingectomies and by 22.1% for neither of both. 38.2%, 1.5% and 24.4% of the participants would use the laparoscopic, transverse and midline laparotomic approach for a surgical staging procedure, respectively. 25.6% (54.7%) of the respondents recommended a hysterectomy in premenopausal (versus postmenopausal) patients with a STIC, 24.4% (88.4%) a bilateral oophorectomy and 50.0% (4.7%) an affected side oophorectomy (all p values < 0.001). Omentectomy, pelvic and para-aortic lymphadenectomy would be performed by 60.5% (64.0%), 9.3% (11.6%) and 9.3% (11.6%) of respondents in premenopausal (versus postmenopausal) patients (all p values > 0.05).
Conclusion
Our survey highlights significant inconsistency in the management of patients with STIC. Prospective data are urgently needed to elucidate the clinical impact of a STIC lesion and its clinical management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Significant inconsistency in the management of patients with STIC resides among German gynaecological oncologists. Prospective data are urgently needed to clarify the impact of a STIC lesion and its clinical management. |
Introduction
Epithelial ovarian cancer (EOC) is the most lethal gynaecological cancer with a 5-year survival rate of 49% [1]. High-grade serous ovarian carcinoma represents the most common histologic type of EOC and the majority of this cancer originate from precursor lesions of the fallopian tube. The latter include epithelia with a p53 signature, secretory cell outgrowths (SCOUT) as well as serous tubal intraepithelial carcinoma (STIC) [2]. STIC is also considered as the precursor lesion of other pelvic (i.e. peritoneal) high-grade serous carcinoma (HGSC) [3,4,5,6].
The lifetime risk for EOC is less than 2% in the general population. In contrast, women with proven BRCA germline mutations have an increased risk for developing ovarian cancer. The cumulative ovarian cancer risk up to the age of 80 years is 44% (95% CI 36%–53%) for BRCA1 and 17% (95% CI 11%–25%) for BRCA2 mutation carriers [7]. For these women, risk-reducing salpingo-oophorectomy (RRSO) is recommended and presents the most effective method of prevention so far [8, 9]. STIC and/or occult carcinoma is detected in approximately 10–15% of these cases [3], and isolated STIC in approximately 2% [10]. In contrast, the incidence of STIC in patients without familial predisposition of EOC is uncertain. A Canadian study reports STIC in 8 out of 9392 women (< 0.01%) with benign diagnoses [11]. Accordingly, a recent published Canadian population-based, retrospective cohort study of patients who underwent opportunistic salpingectomy or a control surgery (hysterectomy alone or tubal ligation) shows that the removal of the fallopian tubes in women at baseline risk for ovarian cancer reduces the risk for EOC [12]. In the future, opportunistic salpingectomies will likely increase in routine surgery as a strategy for EOC prevention.
The precursor lesion STIC is mostly located in the fimbriated end of the fallopian tube and typically demonstrates increased mitotic activity, significant atypia, architectural alterations and aberrant staining pattern for p53 (strong and homogenous, complete loss or cytoplasmic), which indicates the presence of a pathogenic mutation in p53[13]. The pathological work-up is clearly defined and should include the standardized SEE-FIM (Sectioning and Extensively Examining the FIMbria) protocol. The SEE-FIM protocol helps pathologists to better detect these STIC lesions and is nowadays established for RRSO after its first publication in February 2006 [14].
Women with a proven isolated STIC lesion are at substantial risk of developing HGSC. The 5- and 10-year risks to develop peritoneal carcinomatosis after a STIC diagnosis at RRSO are 10.5% (95% CI 6.2–17.2) and 27.5% (95% CI 15.6–43.9) [15], respectively, and predominantly in BRCA1 mutation carriers [16, 17]. The corresponding risks for women without STIC at RRSO are 0.3% (95% CI 0.2–0.6) and 0.9% (95% CI 0.6–1.4) [15].
Besides being a precursor lesion, STICs may already be an indicator for an actually active HGSC of the ovary. Patients with incidental STIC in a low-risk population underwent surgery and three out of seven patients have been upstaged to an HGSC of the ovaries [18]. Therefore, German guidelines recommend informing a patient with a diagnosed STIC lesion about the risk of an already ongoing malignant process and discussing the possibility of surgical staging procedures [19]. However, no further specifications about the extent of the surgery, the methods of pre-surgical diagnostics or imaging are recommended due to the lack of data.
Once a STIC is diagnosed, no established clinical management regarding diagnostics and treatment is available for these patients. Our survey investigates how STIC is diagnosed and treated in gynaecological centres in Germany to critically discuss the actual management.
Materials and methods
A questionnaire with 21 multiple-choice questions was intraprofessionally developed to investigate the management and experience with STIC patients among German gynaecological centres (see supplementary). The online questionnaire was generated using SoSci Survey and was made available to users via www.soscisurvey.de, an open source platform for non-profit research [20]. After the approval of the scientific board of the German working group for ovarian cancer (Arbeitsgemeinschaft Gynäkologische Onkologie (AGO), Organkomission OVAR), a link to the questionnaire was sent to 540 email addresses from all available German gynaecological centres in February 2020. Two reminders were sent in March and April 2020. The list of all German gynaecological centres was provided by the German Society of Gynaecology and Obstetrics (DGGG) in 2006, subsequently updated and used previously for other questionnaire-based analyses. The questionnaire was in German, translated into English and is shown in the supplementary.
Items
Questions included general data concerning the hospitals’ organisational structures and their specific, tumour-related data.
At first, questions comprised the hospitals’ characteristics including, e.g. the number of beds or whether these centres were teaching or university hospitals. Furthermore, information concerning the professional training in gynaecological oncology was collected as well as the numbers of cancer patients treated in each centre (see supplementary, general information).
Detailed information about the pathological analysis and the usage of the standardized SEE-FIM protocol—referred to as ultrastaging in the questionnaire—was obtained subsequently (see supplementary, histological handling). STIC-related data, such as the number of patients diagnosed with a STIC, were gathered. In addition, we created hypothetical questions concerning diagnostics and individual treatment decisions for STIC patients (see supplementary).
These questions included diagnostic procedures, access to surgery, extent of surgery in pre-and postmenopausal women as well as adjuvant chemotherapy.
Statistical analysis
Raw data were obtained through SoSci Survey and listed in Excel files. Data were analysed using SPSS 26.0 (IBM Corp., Armonk, NY, USA) and summarized as means (± standard deviation) or proportions (%). Chi-square test was used to assess significant differences between proportions after exclusion of not available (n/a) answers. A two-sided p value of < 0.05 was considered statistically significant.
Results
Experience of cancer centres
Overall, 131 questionnaires were completed sufficiently to be included within the final statistical analysis (24.3%). Within these 131 returned questionnaires, 75.1% of the questions were answered. Hospitals were categorized in three types. 14.5% of the institutions were university hospitals, 72.5% teaching hospitals and 13% hospitals with no additional designation (Table 1). Most of the participating hospitals (27.5%) possessed more than 60 beds in their department (Table 1). 51.9% were members of the AGO (Table 2), whilst 48.9% of all centres were certified as gynaecological oncology centres and 63.4% were certified as breast cancer centres in accordance with the German cancer society (Deutsche Krebsgesellschaft). Most of the centres treated 201–250 breast cancer patients and 13–24 ovarian cancer patients per year. 27.5% of all participants had at least one physician officially specialized and certified in gynaecological oncology working in their department (Table 2).
Personal experience of participants
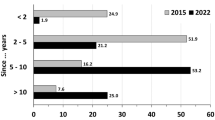
Participants reported long experiences in gynaecological oncology (67.9% more than 10 years, Table 2). 45.8% had treated one to three STIC patients so far (Table 1). Nearly half of all participants (49.6%) had already discussed the topic STIC as part of a workshop in their department (Table 2). The participants regularly used the German evidence-based (S3) guideline for ovarian cancer in their clinical routine within the last year [19] (Table 2). Most centres (75.6%) performed opportunistic salpingectomies; nearly a third of the respondents started with those in 2014 (31.3%).
Diagnostics
Most of the centres implemented the SEE-FIM protocol in 2014 (31.3%). 13.7% applied the protocol for all salpingectomies, 39.7% only for prophylactic salpingectomies and 22.1% for neither.
The participants were asked hypothetically which diagnostic procedures they would perform in case of an isolated STIC lesion. The options included serum CA-125 (38.9%), transvaginal ultrasound (32.1%), magnetic resonance imaging (MRI) of the pelvis (6.1%), computed tomography (CT) of the pelvis (23.7%) and no diagnostics at all (19.8%) (see Fig. 1).
Treatment
With regard to the therapeutical approach, most surgeons would choose a robotic/laparoscopic approach to perform a surgical staging procedure (38.2%), see Fig. 2.
In premenopausal (postmenopausal) STIC patients, 25.6% (54.7%, p < 0.001) of the centres stated to perform a hysterectomy, 24.4% (88.4%, p < 0.001) a bilateral oophorectomy, 50.0% (4.7%, p < 0.001) an affected side oophorectomy. Omentectomy, pelvic and para-aortic lymphadenectomy would be performed by 60.5% (64.0%), 9.3% (11.6%) and 9.3% (11.6%) centres in premenopausal (postmenopausal) STIC patients (all p values > 0.05), respectively, see Fig. 3. Very few participants opted to treat a STIC patient with adjuvant chemotherapy (2.3%).
Discussion
Our survey highlights many inconsistencies in the management of patients with STIC among gynaecological departments in Germany. Even though 49.6% of the centres had a STIC-related training held at their centre, most clinicians had only treated very few patients during their medical career.
A relevant number of participants do not perform an opportunistic salpingectomy routinely during everyday surgery, neither do all of them request the correct pathological examination in accordance with the SEE-FIM protocol, especially for high-risk patients. According to the ESMO–ESGO Consensus, the SEE-FIM protocol should be performed in all risk-reducing prophylactic surgery specimens [21].
Diagnosing STIC is challenging and shows only moderate reproducibility. Therefore, a recently published systematic review suggests not only the use of the SEE-FIM protocol, but also evaluation by a subspecialized pathologist, rational use of immunohistochemical staining, and obtaining a second opinion from a colleague to secure the diagnosis [22].
Recent literature still mostly provides case series of STIC patients with individual diagnostic approaches [15, 23, 24]. Similarly, our findings showed a great variety of approaches as well. Regarding diagnostic procedures, most of the participants would perform a transvaginal ultrasound and control serum CA-125. To date, no effective screening tool exists to monitor STIC patients [25]. Most of the published studies include annual clinical checkups with pelvic ultrasound and in some cases routine evaluation of serum CA-125[17]. BRCA status should additionally be checked in cases of isolated STIC. However, in general, no routine screening for ovarian HGSC should be offered to women of the general population [26, 27].
Peritoneal restaging should be considered in cases of incidentally detected, apparently isolated STIC lesions [21]. A systematic review of the literature in 2018 suggests that a staging procedure as an additional treatment after RRSO and the diagnosis of an isolated STIC is associated with a lower risk of recurrence [24]. A surgical staging for patients with STIC mostly included hysterectomy, omentectomy, pelvic and para-aortic lymph node dissection and peritoneal washing [24]. Interestingly, the routine use of peritoneal biopsies during RRSO does not seem to improve the detection of occult malignancies [28].
Chay et al. suggest that a complete staging surgery should be considered for non-BRCA patients with a STIC lesion as well, since in three out of seven STIC cases, staging surgery led to an upstaging from STIC to HGSC of the ovaries [18]. 38.2% of the centres in our survey would advise a laparoscopic surgery after the diagnosis of STIC, even though guidelines for ovarian cancer recommend a laparotomy for surgical staging. However, data concerning the best approach for the surgical staging in patients with STIC are lacking so far. A systematic Cochrane review could not help to quantify the value of laparoscopy for the management of early stage ovarian cancer as routine clinical practice [19, 29]. Furthermore, a cohort study of the AGO OVAR regarding ovarian borderline tumours could not show any significant impact of the initial surgical approach on recurrence either [30].
Remarkably, the completeness of surgical staging in patients with early ovarian cancer is significantly associated with better outcomes compared to incomplete surgical staging procedures [31]. This association has not been proven for patients with a STIC lesion. We found significant differences in strategies regarding the extent of surgical procedures in pre- versus postmenopausal women after the diagnosis of STIC. Most centres would perform oophorectomies for the affected side only in premenopausal women, while a bilateral oophorectomy would be performed in postmenopausal women. In view of these results, the questionnaire might be modified in the future and stratify the women by the status of the family planning rather than their menopausal status. No data are yet available concerning STIC patients and the effects of delayed oophorectomy to prevent early onset of menopause and non-cancer-related morbidity, but these questions are currently being addressed by an ongoing clinical trial (ClinicalTrials.gov identifier: NCT04294927, ISRCTN 25,173,360, and ClinicalTrials.gov identifier: NCT04251052).
Patrono and colleagues reviewed 78 STIC cases, of whom 16.4% received adjuvant chemotherapy [23]. In our survey, adjuvant chemotherapy was rarely recommended by the centres. In general, adjuvant chemotherapy for intraepithelial neoplasia with negative washing is not advised any longer [21].
Routine surveillance for every patient with STIC is recommended for the next years, because the time from STIC to invasive cancer has been suggested to be approximately 7 years and has guided the recommendation for RRSO at 35–40 years of age in BRCA1 patients [32].
To the best of our knowledge, we performed the first survey regarding the clinical management of patients with STIC in Germany so far. The response rate to our study was moderate but within the range of health care professionals’ surveys [33]. Unfortunately, many of the participants only finished the first part of the survey and did not take part in the more detailed case-related questions. A further limitation is the low experience of the centres with STIC patients, since most of the respondents had treated only one to three STIC cases up to date due to its low incidence. Furthermore, a questionnaire with hypothetical questions should be interpreted with caution because clinical decision in the real world might be different. The questionnaire just comprised 21 questions and some important questions were not asked, e.g. if only gynaecological pathologists performed the histological examination. It remains unclear if the centres have their own clinical standard with a predefined protocol concerning diagnostics and treatment of STIC. Therefore, future studies should be performed to update the clinical day routine prospectively.
Our survey demonstrates the lack of consistency in the management of patients with STIC in Germany. It underlines the need for more information about isolated STIC, especially regarding the best approach and extent of the surgical staging as well as the risk for isolated lymph node metastasis without peritoneal carcinomatosis, in general. A prospective register collecting clinical data of STIC patients might be helpful to evaluate the clinical courses of the disease and to identify important diagnostic and therapeutic tools. This may establish an evidence-based strategy and will finally lead to a validated guideline for diagnostic procedures and treatment of women with a STIC to support gynaecological oncologists in their daily practice. Additionally, interdisciplinary educational programmes should be established to increase the awareness.
Data availability statement
All data generated or analysed during this study are included in this article and its tables and figures. Further enquiries can be directed to the corresponding author.
References
Siegel RL et al (2022) Cancer statistics, 2022. CA Cancer J Clin 72(1):7–33
Meserve EEK, Brouwer J, Crum CP (2017) Serous tubal intraepithelial neoplasia: the concept and its application. Mod Pathol 30(5):710–721
Li HX et al (2014) Advances in serous tubal intraepithelial carcinoma: correlation with high grade serous carcinoma and ovarian carcinogenesis. Int J Clin Exp Pathol 7(3):848–857
Kurman RJ, Shih I-M (2010) The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol 34(3):433–443
Piek JM et al (2001) Dysplastic changes in prophylactically removed fallopian tubes of women predisposed to developing ovarian cancer. J Pathol 195(4):451–456
Carlson JW et al (2008) Serous tubal intraepithelial carcinoma: its potential role in primary peritoneal serous carcinoma and serous cancer prevention. J Clin Oncol 26(25):4160–4165
Kuchenbaecker KB et al (2017) Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 317(23):2402–2416
Eleje GU et al (2018) Risk-reducing bilateral salpingo-oophorectomy in women with BRCA1 or BRCA2 mutations. Cochrane Database Syst Rev 8:012464
Rebbeck TR, Kauff ND, Domchek SM (2009) Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst 101(2):80–87
Wethington SL et al (2013) Clinical outcome of isolated serous tubal intraepithelial carcinomas (STIC). Int J Gynecol Cancer 23(9):1603–1611
Samimi G et al (2018) Population frequency of serous tubal intraepithelial carcinoma (STIC) in clinical practice using SEE-Fim protocol. JNCI Cancer Spectr 2(4):pky061
Hanley GE et al (2022) Outcomes from opportunistic salpingectomy for ovarian cancer prevention. JAMA Netw Open 5(2):e2147343
Bachert SE et al (2020) Serous tubal intraepithelial carcinoma: a concise review for the practicing pathologist and clinician. Diagnostics (Basel) 10(2):102
Medeiros F et al (2006) The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol 30(2):230–236
Steenbeek MP et al (2022) Risk of peritoneal carcinomatosis after risk-reducing salpingo-oophorectomy: a systematic review and individual patient data meta-analysis. J Clin Oncol 40:1879–1891
Harmsen MG et al (2018) Peritoneal carcinomatosis after risk-reducing surgery in BRCA1/2 mutation carriers. Cancer 124(5):952–959
Linz VC, Loewe A, van der Ven J, Hasenburg A, Battista MJ (2022) Incidence of pelvic high-grade serous carcinoma after isolated STIC diagnosis: a systematic review of the literature. Front Oncol 12:951292
Chay WY et al (2016) Outcomes of incidental fallopian tube high-grade serous carcinoma and serous tubal intraepithelial carcinoma in women at low risk of hereditary breast and ovarian cancer. Int J Gynecol Cancer 26(3):431–436
Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, D.K., AWMF), S3-Leitlinie Diagnostik, Therapie und Nachsorge maligner Ovarialtumoren Langversion 4.0. AWMF-Registernummer: 032/035OL, 03/2020. Version 4.0.
Leiner DJ. SoSci Survey. 2019. Available at https://www.soscisurvey.de. p. [Computer software]
Colombo N et al (2019) ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Int J Gynecol Cancer 29:728
Bogaerts JMA et al (2022) Recommendations for diagnosing STIC: a systematic review and meta-analysis. Virchows Arch 480(4):725–737
Patrono MG et al (2015) Clinical outcomes in patients with isolated serous tubal intraepithelial carcinoma (STIC): a comprehensive review. Gynecol Oncol 139(3):568–572
Van der Hoeven NMA et al (2018) Outcome and prognostic impact of surgical staging in serous tubal intraepithelial carcinoma: a cohort study and systematic review. Clin Oncol (R Coll Radiol) 30(8):463–471
Poon C et al (2016) Incidence and characteristics of unsuspected neoplasia discovered in high-risk women undergoing risk reductive bilateral salpingooophorectomy. Int J Gynecol Cancer 26(8):1415–1420
Menon U et al (2021) Ovarian cancer population screening and mortality after long-term follow-up in the UK collaborative trial of ovarian cancer screening (UKCTOCS): a randomised controlled trial. Lancet 397(10290):2182–2193
Buys SS et al (2011) Effect of screening on ovarian cancer mortality: the prostate, lung, colorectal and ovarian (PLCO) cancer screening randomized controlled trial. JAMA 305(22):2295–2303
Marchetti C et al (2022) Risk reducing surgery with peritoneal staging in BRCA1–2 mutation carriers. A prospective study. Eur J Surg Oncol 48:2539
Medeiros LR et al (2008) Laparoscopy versus laparotomy for FIGO stage I ovarian cancer. Cochrane Database Syst Rev 4:CD005344
du Bois A et al (2013) Borderline tumours of the ovary: a cohort study of the arbeitsgmeinschaft gynakologische onkologie (AGO) study group. Eur J Cancer 49(8):1905–1914
Trimbos B et al (2010) Surgical staging and treatment of early ovarian cancer: long-term analysis from a randomized trial. J Natl Cancer Inst 102(13):982–987
Labidi-Galy SI et al (2017) High grade serous ovarian carcinomas originate in the fallopian tube. Nat Commun 8(1):1093
Meyer VM et al (2022) Global overview of response rates in patient and health care professional surveys in surgery: a systematic review. Ann Surg 275(1):e75–e81
Acknowledgements
The authors would like to thank the AGO OVAR and all participating centres for their support.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Conceptualization: JVDV, VCL, AH and MJB; design: JVDV, VCL, AH and MJB; data acquisition: JVDV, VCL and MJB; analysis and interpretation: JVDV, VCL and MJB; writing—original draft of the manuscript: VCL and JVDV, writing—review and editing: VCL, JVDV, KA, MWS, AL, SlK, MS, StK, BS, JS, AH and MJB. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
V.C.L., J.V.D.V, A.L., M.W.S., B.S., J.S. and M.J.B. have no relevant financial or non-financial interests to disclose. M.S. reports personal fees from AstraZeneca, BioNTech, Daiichi Sankyo, Eisai, Lilly, MSD, Novartis, Pantarhei Bioscience, Pfizer, Roche, and SeaGen outside the submitted work; institutional research funding from AstraZeneca, BioNTech, Eisai, Genentech, German Breast Group, Novartis, Palleos, Pantarhei Bioscience, Pierre Fabre, and SeaGen. In addition, M.S. has a patent for EP 2390370 B1 issued and a patent for EP 2951317 B1 issued. K.A. reports personal fees from Eisai, Roche Pharma AG, MSD, AstraZeneca and Clovis Oncology. Sl.K. received speaker honoraria from Roche and Novartis, research funding from Novartis and travel reimbursement from PharmaMar and Novartis. St.K. received honoraria for advisory boards and/or educational activities (outside submitted work) from Roche, Astra Zeneca, GSK, Myriad, Clovis, MSD. A.H. reports honoraria and expenses from AstraZeneca, FBA Frauenärzte BundesAkademie GmbH, KlarigoVerlag, MedConcept, Med public GmbH, Med update GmbH, Medicultus, Pfizer, Promedicis GmbH, Pierre Fabre Pharma GmbH, Softconsult, Roche Pharma AG, Streamedup! GmbH, Tesaro Bio Germany GmbH. A.H. is consultant to PharmaMar, Promedicis GmbH, Pierre Fabre Pharma GmbH, Roche Pharma AG and Tesaro Bio Germany GmbH and has received funded research from Celgene. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical approval
This is a questionnaire-based survey and no ethical approval is required.
Patient consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van der Ven, J., Linz, V.C., Anic, K. et al. A questionnaire-based survey on the diagnostic and therapeutic approaches for patients with STIC in Germany. Arch Gynecol Obstet 308, 527–534 (2023). https://doi.org/10.1007/s00404-023-06919-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-023-06919-8