Abstract
Aims
The various diagnostic criteria for polycystic ovary syndrome (PCOS) raised problem for PCOS research worldwide. PCOS has been demonstrated to be significantly associated with immune response. We aimed to identify several immune-related biomarkers and construct a nomogram model for diagnosis in PCOS.
Methods
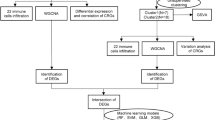
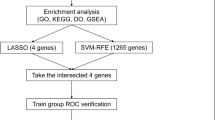
The mRNA expression data were downloaded from Gene Expression Omnibus (GEO) database. Significant immune-related genes were identified to be the biomarkers for the diagnosis of PCOS using random forest model (RF), support vector machine model (SVM) and generalized linear model (GLM). The key biomarkers were selected from the optimal model and were utilized to construct a diagnostic nomogram. Receiver operating characteristic (ROC) curves was used to evaluate diagnostic ability of nomogram. Moreover, the relative proportion of 22 immune cell types was calculated by CIBERSORT algorithm.
Results
Four immune-related biomarkers (cAMP, S100A9, TLR8 and IL6R) were demonstrated to be highly expressed in PCOS. The nomogram constructed on the ground of the four key biomarkers showed perfect performance in diagnosis of PCOS, whose AUC were greater than 0.7. Higher infiltrating abundance of neutrophils, resting NK cells and activated dendritic cells were observed in PCOS and were tightly associated with the four key biomarkers.
Conclusions
This study identified several immune-related diagnostic biomarkers for PCOS patients. The diagnostic nomogram constructed based the biomarkers provide a theory foundation for clinical application. Multiple immune cells were associated with the expression of these four biomarkers and might played a vital role in the procession of PCOS.






Similar content being viewed by others
Data availability
Not applicable.
References
Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF (2009) The androgen excess and PCOS society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril 91(2):456–488
Ganie MA, Vasudevan V, Wani IA, Baba MS, Arif T, Rashid A (2019) Epidemiology, pathogenesis, genetics & management of polycystic ovary syndrome in India. Indian J Med Res 150(4):333–344
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 81(1):19–25
Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R (2016) Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril 106(1):6–15
Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, Welt CK (2013) Diagnosis and treatment of polycystic ovary syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 98(12):4565–4592
Witchel SF, Burghard AC, Tao RH, Oberfield SE (2019) The diagnosis and treatment of PCOS in adolescents: an update. Curr Opin Pediatr 31(4):562–569
Escobar-Morreale HF, Luque-Ramírez M, San Millán JL (2005) The molecular-genetic basis of functional hyperandrogenism and the polycystic ovary syndrome. Endocr Rev 26(2):251–282
Zhang T, Tian F, Huo R, Tang A, Zeng Y, Duan YG (2017) Detection of dendritic cells and related cytokines in follicular fluid of patients with polycystic ovary syndrome. Am J Reprod Immunol. https://doi.org/10.1111/aji.12717
Liu Y, Liu H, Li Z, Fan H, Yan X, Liu X, Xuan J, Feng D, Wei X (2021) The release of peripheral immune inflammatory cytokines promote an inflammatory cascade in pcos patients via altering the follicular microenvironment. Front Immunol 12:685724
González F (2012) Inflammation in polycystic ovary syndrome: underpinning of insulin resistance and ovarian dysfunction. Steroids 77(4):300–305
Hu C, Pang B, Ma Z, Yi H (2020) Immunophenotypic profiles in polycystic ovary syndrome. Mediators Inflamm 2020:5894768
Yi W, Li X, Chen K, Li J, Chen K, Pan A (2020) Effect of rNA interference on Oatp3a1 gene expression on biological characteristics and immune factors of ovarian granulosa cells in rats with PCOS. Am J Transl Res 12(8):4659–4668
Roh EY, Yoon JH, Song EY, Kim JJ, Hwang KR, Seo SH, Shin S (2017) Single nucleotide polymorphisms in the TGF-β1 gene are associated with polycystic ovary syndrome susceptibility and characteristics: a study in Korean women. J Assist Reprod Genet 34(1):139–147
Zhao J, Xu J, Wang W, Zhao H, Liu H, Liu X, Liu J, Sun Y, Dunaif A, Du Y, Chen ZJ (2018) Long non-coding RNA LINC-01572:28 inhibits granulosa cell growth via a decrease in p27 (Kip1) degradation in patients with polycystic ovary syndrome. EBioMedicine 36:526–538
Kenigsberg S, Bentov Y, Chalifa-Caspi V, Potashnik G, Ofir R, Birk OS (2009) Gene expression microarray profiles of cumulus cells in lean and overweight-obese polycystic ovary syndrome patients. Mol Hum Reprod 15(2):89–103
Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD (2012) The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 28(6):882–883
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK (2015) limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43(7):e47–e47
Wang L, Cao C, Ma Q, Zeng Q, Wang H, Cheng Z, Zhu G, Qi J, Ma H, Nian H (2014) RNA-seq analyses of multiple meristems of soybean: novel and alternative transcripts, evolutionary and functional implications. BMC Plant Biol 14(1):1–19
Langfelder P, Horvath S (2008) WGCNA: an r package for weighted correlation network analysis. BMC Bioinformatics 9:559
da Huang W, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4(1):44–57
Pan X, Jin X, Wang J, Hu Q, Dai B (2021) Placenta inflammation is closely associated with gestational diabetes mellitus. Am J Transl Res 13(5):4068–4079
Tibshirani R (1997) The lasso method for variable selection in the cox model. Stat Med 16(4):385–395
Chen B, Khodadoust MS, Liu CL, Newman AM, Alizadeh AA (2018) Profiling tumor infiltrating immune cells with CIBERSORT. Methods Mol Biol 1711:243–259
Shabani F, Farasat A, Mahdavi M, Gheibi N (2018) Calprotectin (S100A8/S100A9): a key protein between inflammation and cancer. Inflamm Res 67(10):801–812
Li H, Huang X, Chang X, Yao J, He Q, Shen Z, Ji Y, Wang K (2020) S100–A9 protein in exosomes derived from follicular fluid promotes inflammation via activation of NF-κB pathway in polycystic ovary syndrome. J Cell Mol Med 24(1):114–125
Bi X, Zhai Z, Wang S (2019) Identification of the key pathways and genes related to polycystic ovary syndrome using bioinformatics analysis. Gen Physiol Biophys 38(3):205–214
Wu X, Yu T, Ji N, Huang Y, Gao L, Shi W, Yan Y, Li H, Ma L, Wu K, Wu Z (2019) IL6R inhibits viability and apoptosis of pancreatic beta-cells in type 2 diabetes mellitus via regulation by miR-22 of the JAK/STAT signaling pathway. Diabetes Metab Syndr Obes 12:1645–1657
Høgdall D, O’Rourke CJ, Dehlendorff C, Larsen OF, Jensen LH, Johansen AZ, Dang H, Factor VM, Grunnet M, Mau-Sørensen M, Oliveira D, Linnemann D, Boisen MK, Wang XW, Johansen JS, Andersen JB (2020) Serum IL6 as a prognostic biomarker and IL6R as a therapeutic target in biliary tract cancers. Clin Cancer Res 26(21):5655–5667
Qin Z, Wang PY, Wan JJ, Zhang Y, Wei J, Sun Y, Liu X (2020) MicroRNA124-IL6R mediates the effect of nicotine in inflammatory bowel disease by shifting Th1/Th2 balance toward Th1. Front Immunol 11:235
Zhang H, Kong Q, Wang J, Jiang Y, Hua H (2020) Complex roles of cAMP-PKA-CREB signaling in cancer. Exp Hematol Oncol 9(1):32
Sirotkin AV, Balazi A, Chrenek P (2014) The cAMP analogue, dbcAMP, affects rabbit ovarian cell proliferation, apoptosis, release of steroids and response to hormones. Folia Biol (Krakow) 62(3):211–218
Huang H, Wang Y, Kandpal M, Zhao G, Cardenas H, Ji Y, Chaparala A, Tanner EJ, Chen J, Davuluri RV, Matei D (2020) FTO-Dependent N (6)-methyladenosine modifications inhibit ovarian cancer stem cell self-renewal by blocking camp signaling. Cancer Res 80(16):3200–3214
Raker VK, Becker C, Steinbrink K (2016) The cAMP pathway as therapeutic target in autoimmune and inflammatory diseases. Front Immunol 7:123
Liew PX, Kubes P (2019) The neutrophil’s role during health and disease. Physiol Rev 99(2):1223–1248
Yilmaz MA, Duran C, Basaran M (2016) The mean platelet volume and neutrophil to lymphocyte ratio in obese and lean patients with polycystic ovary syndrome. J Endocrinol Invest 39(1):45–53
Bryceson YT, March ME, Ljunggren HG, Long EO (2006) Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood 107(1):159–166
Morante-Palacios O, Fondelli F, Ballestar E, Martínez-Cáceres EM (2021) Tolerogenic dendritic cells in autoimmunity and inflammatory diseases. Trends Immunol 42(1):59–75
Li L, Liu X, Sanders KL, Edwards JL, Ye J, Si F, Gao A, Huang L, Hsueh EC, Ford DA, Hoft DF, Peng G (2019) TLR8-mediated metabolic control of human treg function: a mechanistic target for cancer immunotherapy. Cell Metab 29(1):103-123.e5
Lian J, Yue Y, Yu W, Zhang Y (2020) Immunosenescence: a key player in cancer development. J Hematol Oncol 13(1):151
Chalaris A, Rabe B, Paliga K, Lange H, Laskay T, Fielding CA, Jones SA, Rose-John S, Scheller J (2007) Apoptosis is a natural stimulus of IL6R shedding and contributes to the proinflammatory trans-signaling function of neutrophils. Blood 110(6):1748–1755
Pan X, Jin X, Wang J, Hu Q, Dai B (2021) Placenta inflammation is closely associated with gestational diabetes mellitus. Am J Translational Res 13(5):4068–4079
Funding
This work was supported by National Natural Science Foundation of China (Program No. 82172714), Natural Science Foundation of Shanghai (Program No. 20ZR1443900).
Author information
Authors and Affiliations
Contributions
JQ carried out the Conception and design of the research, XT and MQ participated in the Acquisition of data. XT and JW carried out the Analysis and interpretation of data. ZC and KL participated in the design of the study and performed the statistical analysis. JQ and BL conceived of the study, and participated in its design and coordination and helped to draft the manuscript and revision of manuscript for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qu, J., Li, B., Qiu, M. et al. Discovery of immune-related diagnostic biomarkers and construction of diagnostic model in varies polycystic ovary syndrome. Arch Gynecol Obstet 306, 1607–1615 (2022). https://doi.org/10.1007/s00404-022-06686-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-022-06686-y