Abstract
Objective
Morular metaplasia (MM) is a benign epithelial metaplasia that sometimes appears in atypical endometrial hyperplasia (AEH) and endometrioid endometrial carcinoma (EEC). However, the clinical implications of MM for fertility-preserving treatment in AEH and EEC patients are unclear. This study investigated the clinical features and impact of MM on the efficacy of fertility-preserving treatment.
Methods
We retrospectively studied 427 AEH and EEC patients who received fertility-preserving treatment. Clinical features, treatment efficacy, and onco-fertility results were compared between patients with and without MM.
Results
MM appeared in 147 of 427 (34.4%) patients. Among them, 49 (33.3%) had MM only before treatment (BEF group), 32 (21.8%) had sustained MM before and during treatment (SUS group), and 66 (44.9%) had MM only during treatment (DUR group). The BEF group had a higher 12-month CR rate (98.0% vs 85.7%, p = 0.017) and shorter therapeutic duration to achieve CR (4.0 vs 5.7 months, p = 0.013) than the non-MM group had. In comparison with the non-MM group, the SUS and DUR groups had a lower CR rate after 7 months of treatment (SUS vs non-MM, 37.5% vs 61.1%, p = 0.010; DUR vs non-MM 33.3% vs. 61.1%, p < 0.001), and a longer median therapeutic duration to achieve CR (SUS vs non-MM, 7.6 vs. 4.0 months, p = 0.037; DUR vs non-MM, 7.9 vs. 4.0 months, p < 0.001).
Conclusion
Appearance of MM only before treatment was positively correlated with outcome of fertility-preserving treatment, while sustained MM or appearance of MM only during treatment implied poorer outcome of fertility-preserving treatment in AEH and EEC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Morular metaplasia (MM) is a benign epithelial metaplasia [1,2,3] that arises from immature squamous epithelial cell differentiation [4, 5]. As a rare condition of epithelial metaplasia, MM presents as morphologically rounded, well-circumscribed aggregations of uniform, oval, or spindle-shaped cell clusters [6, 7]. MM might sometimes appear in the endometrium and is almost always associated with focal, complex endometrial glandular lesions [1, 8]. Studies have shown that MM in endometrial lesions is associated with benign diseases or relatively inert malignancy [3, 6, 8, 9]. Although the mechanisms of occurrence of MM in the endometrium are not sufficiently clear, it has been reported that the occurrence of MM is associated with chronic endometritis, submucosal leiomyoma, irradiation, exogenous hormone therapy, and intrauterine devices [1, 2].
MM is also found in atypical endometrial hyperplasia (AEH) or early-stage endometrioid endometrial cancer (EEC) lesions receiving fertility-preserving treatment. This pathological phenomenon appears either before or during progestin-based treatment in endometrial lesions. However, the clinical implications of the appearance of MM are not clear in these patients receiving fertility-preserving treatment.
In this study, we retrospectively analyzed AEH and EEC patients who received fertility-preserving treatment at the Obstetrics and Gynecology Hospital of Fudan University. Clinical characteristics and treatment outcomes of patients with MM before and/or during fertility-preserving treatment were analyzed and compared with those in patients without MM. Appearance of MM in endometrial lesions only before fertility-preserving treatment was associated with higher complete response (CR) rate and shorter treatment duration to achieve CR in AEH and EEC patients compared with those without MM.
Materials and methods
Study population
This was a retrospective study of 590 consecutive patients (441 AEH and 149 EEC) who received fertility-preserving treatment at the Obstetrics and Gynecology Hospital of Fudan University between March 2013 and October 2019 (Fig. 1). Because it has been reported that the appearance of MM is correlated with hormone use, we excluded 122 patients who received progestin treatment for > 1 month before the first endometrial evaluation at our hospital. Another 41 patients with papillary hyperplasia in endometrial lesions were also excluded because this pathological type might also have some impact on fertility-preserving treatment. Ultimately, 427 patients (323 AEH and 104 EEC grade 1) were included. All the patients received standardized evaluation and treatment, and patient information was prospectively collected and recorded during treatment and follow-up. The study was approved by the Ethics Committee of Obstetrics and Gynecology Hospital of Fudan University.
The inclusion and exclusion criteria for fertility-sparing treatment followed National Comprehensive Cancer Network guidelines [10] and included: (1) histologically proven AEH, or well-differentiated EEC grade 1 without myometrial invasion; (2) no signs of suspicious extrauterine involvement on enhanced magnetic resonance imaging (MRI), enhanced computed tomography (CT), or ultrasound; (3) age < 45 years; (4) strong willingness to preserve fertility; (5) no contraindication for progestin treatment or pregnancy; (6) not pregnant; and (7) good compliance for treatment. Written informed consent was obtained from all patients before initiating treatment. Patients with MM in endometrial lesions before and/or during fertility-preserving treatment were included in the study group. Patients with AEH and/or EEC grade 1 only were included as the non-MM group.
Pathological diagnosis
All patients were pathologically diagnosed through dilation and curettage with or without hysteroscopy. Pathological diagnosis was confirmed by two experienced gynecological pathologists according to the World Health Organization pathological classifications (2014). If their opinions differed, a seminar was held in the Pathology Department to achieve a final diagnosis. We performed another hysteroscopy within 1 month after initial pathological diagnosis if the patient was diagnosed by dilation and curettage without hysteroscopy.
Diagnosis of morular metaplasia was based on Blaustein’s Pathology of the Female Genital Tract. Briefly, MM is composed of rounded aggregates or syncytial sheets of cells that often fill the glandular lumina. The constituent cells have central bland, round, ovoid, or spindle shapes, evenly spaced nuclei, and sometimes show small nucleoli [2].
Fertility-preserving treatment and evaluation of treatment outcomes
Fertility-preserving treatment was initiated as soon as comprehensive evaluation was completed, and the multidisciplinary team judged the patient suitable for treatment. Therapeutic regimens were decided by physicians. Most patients received oral megestrol acetate (MA) at 160 mg/day with or without metformin (500 mg, three times daily). The other patients were treated with a levonorgestrel-releasing intrauterine system (LNG-IUS), ethinylestradiol cyproterone (Diane-35), or gonadotropin-releasing hormone analog (GnRH-a) plus letrozole. A comprehensive hysteroscopic evaluation was performed every 3 months during treatment to evaluate therapeutic efficacy [11]. Endometrial lesions were removed under hysteroscopy, and an endometrial biopsy was randomly performed if no obvious lesion was found.
Treatment response was evaluated histologically using specimens obtained during each hysteroscopy. Complete response (CR) was defined as no hyperplasia or cancerous lesion. Partial response (PR) was defined as pathological improvement. Stable disease (SD) was defined as persistence of the initially diagnosed lesion. Progressive disease (PD) was defined as evidence of EC in patients with AEH, or evidence of more severe pathological findings, myometrial invasion, or extrauterine metastasis in EEC patients.
Once the patient achieved CR, the same regimen was continued for another 2–3 months for consolidation. Hysteroscopy was performed 3 months after the first CR, for confirmation. The therapeutic duration to achieve CR was calculated from the time of initiating treatment to the time of first pathological CR diagnosis, if no hyperplasia or cancerous lesion was found in two consecutive hysteroscopic evaluations. All patients desiring fertility were encouraged to receive assisted reproductive treatments such as in vitro fertilization after CR. Low-dose progestin, oral contraceptive pills, or LNG-IUS was used to prevent recurrence in patients who did not have a recent parental plan. Hysterectomy was strongly recommended for patients who had SD for 6 months, PR for 9 months, or PD at any time during treatment. For patients who refused hysterectomy, alternative treatments including Diane-35 (one pill/day for 21 days of a 28-day cycle) combined with metformin (500 mg, three times daily), LNG-IUS insertion, or intramuscular injection of GnRH-a were given according to the recommendations of a multidisciplinary team.
Patients were followed up every 3–6 months after CR. Ultrasound evaluation was made at each follow-up visit and endometrial biopsy using Pipelle was performed every 6 months during follow-up. Patients were followed up until October 2020. The median follow-up time, from initial treatment in our center to the last follow-up, was 26.2 months (range 3.2–90.3 months). The median follow-up time from the date of achieving CR to the last follow-up was 24.3 months (range 3.1–76.9 months).
Data collection
General information about the patients, including age, weight, height, basic blood pressure, and comorbidities (e.g., hypertension or diabetes) was collected before any treatment was given. Blood samples before initiating fertility-preserving treatment were collected and tested for fasting blood glucose, fasting insulin, and lipid profiles. All blood samples were collected and examined in the laboratory of the Obstetrics and Gynecology Hospital as previously described [12]. Body mass index (BMI) was calculated as weight (kg)/height2 (m2). BMI ≥ 25 kg/m2 was considered as overweight [13]. Insulin resistance (IR) was estimated using the homeostasis model assessment of insulin resistance (HOMA-IR). The HOMA-IR index was calculated as fasting blood glucose (mmol/L) × fasting insulin (μU/mL)/22.5 [14]. When HOMA-IR was ≥ 2.96, we defined the patient as having IR [15]. The diagnostic criteria for metabolic syndrome (MS) have been described previously [12]. Symptoms of chronic estrogen stimulation also were collected, including menometrorrhagia, prolonged menstruation, irregular menses, early menarche, amenorrhea, polycystic ovary syndrome (PCOS). When the time of menarche was 11 years old or less, we defined the patient as early menarche [16]. Diagnosis of PCOS was based on Rotterdam Consensus Criteria [17].
Statistical analysis
All descriptive data are presented as mean and SD for data with a Gaussian distribution and as median plus range for non-Gaussian distributed data. The categorical variables are presented as frequency and percentage. Numerical variables were analyzed using Student’s t test or Mann–Whitney U test. The chi-square test was used to analyze categorical variables, except if the expected frequency was < 5; when this was the case, Fisher’s exact test or likelihood-ratio chi-square test was used. Therapeutic duration was estimated by the Kaplan–Meier method and compared between groups using the log-rank test. A Cox regression model was used for univariate and multivariate analyses of the relationship between covariates and CR during fertility-preserving treatment. A logistic regression model was used to measure the association between morular metaplasia and risk factors in multivariate analyses. SPSS version 23.0 (IBM, Armonk, NY, USA) was used for all statistical analyses, and two-tailed p values < 0.05 were considered statistically significant.
Results
General characteristics of patients with or without MM
Totally, twenty-four patients (24/427, 5.6%) were lost to follow-up till October 2020, including 12 patients who cannot be contacted, 4 patients who did not come back to our clinic after being contacted and 10 patients who rejected to be followed up. Among those patients, 7 (7/147, 4.8%) were in MM group and 17 (17/280, 6.1%) were in non-MM group.
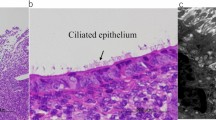
MM appeared in 147 (34.4%) patients. Among them, 49 (33.3%) had MM only before treatment that disappeared after initiation of treatment (BEF group); 32 (21.8%) had sustained MM before and during treatment (SUS group); and 66 (44.9%) had MM only during treatment (DUR group). The remaining 280 (65.6%) patients comprised the non-MM group who had only AEH or EEC in endometrial lesions, without any other specific pathological phenomena. In the DUR group, median duration from initiation of treatment to the appearance of MM was 4.2 months (range 1.0–21.4 months). Representative images of MM are shown in Fig. 2. Expression of progestin receptors in MM in endometrial lesions was all negative.
Morular metaplasia (indicated by the square) in endometrial lesions and its immuno-histochemical staining for estimation of ER (b, e, h, k) and PR (c, f, i, l). Expression of PR was all negative among morular metaplasia. a–c Morular metaplasia appeared only before progestin treatment. d–i Sustained morular metaplasia. (d–f, before progestin treatment; g–i, during progestin treatment). j–l Morular metaplasia appeared only during progestin treatment. (a, d, g, j: original magnification × 10; other photographs: magnification × 20). ER, estrogen receptor; PR, progesterone receptor
General characteristics of patients with and without MM are presented in Table 1. Patients in the SUS group (age 29.0 years, p = 0.001) and DUR group (age 31.0 years, p = 0.022) were both younger than those in the non-MM group (age 33.0 years). Follicle-stimulating hormone (FSH) in the BEF group was higher than in the non-MM group (6.87 vs 6.27 mIU/mL, p = 0.048). There were no other significant differences between the SUS and DUR groups and non-MM group. More patients in the DUR group had metabolic disorders than those in the BEF group, which was demonstrated by higher BMI (25.80 vs 23.01 kg/m2, p = 0.003), and more patients with MS (54.5% vs 32.7%, p = 0.020) and hypertension (28.8% vs 12.2%, p = 0.033).
370 patients received MA with or without metformin while 57 patients received other regimens including 54 patients receiving LNG-IUS, 1 patient receiving Diane-35 and 2 patients receiving GnRH-a plus letrozole. The reason of using Diane-35 on the only one patient was her typical symptoms of PCOS and starting Diane-35 for less than 1 month before coming to our center. One patient received GnRH-a plus letrozole because of her high risk of thrombosis and hepatic dysfunction. The other because of her thrombophlebitis of left forearm.
No significant differences were found in treatment regimens, disease prevention methods, preparation for pregnancy, or conservative treatment regimens among these groups.
Implications of MM for fertility-preserving treatment outcome
To investigate the clinical implications of MM for fertility-preserving treatment in AEH and EEC patients, we analyzed the CR rate and therapeutic duration to achieve CR in patients with or without MM (Table 2, Fig. 3a, b). The BEF group had better conservative treatment outcome compared with the non-MM group, while the SUS and DUR groups had worse treatment outcome compared with the non-MM group. The BEF group had a significantly higher cumulative CR rate after 10 months of treatment compared with the non-MM group (91.8% vs. 80.0%, p = 0.048). There was a similar tendency in cumulative CR rate after 12 months of treatment (98.0% vs. 85.7%, p < 0.017). The median therapeutic duration to achieve CR in the BEF group was significantly shorter than in the non-MM group (4.0 vs 5.7 months, p = 0.013). In contrast, in comparison with the non-MM group, the SUS and DUR groups had a lower CR rate after 7 months of treatment (SUS vs non-MM, 37.5% vs 61.1%, p = 0.010; DUR vs non-MM, 33.3% vs 61.1%, p < 0.001). The SUS and DUR groups both had a longer median therapeutic duration to achieve CR than the non-MM group had (SUS vs non-MM, 7.6 vs 4.0 months, p = 0.037; DUR vs non-MM, 7.9 vs 4.0 months, p < 0.001).
Cumulative CR rate in AEH and EEC patients. a Cumulative CR rate in patients with and without morular metaplasia. b Cumulative CR rate among three subgroups of patients with morular metaplasia and without morular metaplasia. CR complete response, MM morular metaplasia, AEH atypical endometrial hyperplasia, EEC endometrioid endometrial carcinoma, BEF MM before treatment group, SUS sustained MM group, DUR MM during treatment group
The pregnancy and relapse rates are shown in Table 2. Among the 402 patients who achieved CR, 241 planned for parenthood. At the time of last follow-up, 80/241 (33.2%) patients achieved at least one pregnancy, including 52/148 (35.1%) patients without and 28/93 (30.1%) with MM. The live birth rate was 23.6% (35/148 cases) and 22.6% (21/93 cases) among patients without or with MM, respectively. No significant differences were found among the groups with or without MM in terms of pregnancy and live birth rates.
Among the 402 patients who achieved CR, 46 (11.4%) relapsed within 24 months after CR, including 28/262 (10.7%) without and 18/140 (12.9%) with MM. The median interval from CR to recurrence was 13.1 months (range 2.5–58.8 months) for patients without and 14.0 months (range 4.9–37.8 months) for patients with MM. No significant differences in relapse were found among the groups with or without MM.
Factors associated with CR
We performed univariate and multivariate analyses to determine factors related to CR in AEH and EEC patients receiving fertility-preserving treatment (Fig. 4). Univariate Cox regression analysis showed that MM during treatment [hazard ratio (HR) 0.68, 95% confidence interval (CI) 0.47–0.79, p = 0.045], sustained MM (HR 0.59, 95% CI 0.45–0.79, p < 0.001), BMI ≥ 25 kg/m2 (HR 0.73, 95% CI 0.60–0.89, p = 0.002), IR (HR 0.76, 95% CI 0.62–0.93, p = 0.007), and lesion size > 2 cm (HR 0.59, 95% CI 0.48–0.72, p < 0.001) were correlated with lower CR rate. In contrast, AEH (HR 1.26, 95% CI 1.0–1.59, p = 0.047) and MM before treatment (HR 1.51, 95% CI 1.11–2.05, p = 0.009) were correlated with higher CR rate.
Uni- and multi-variate analyses for factors associated with complete response according to Cox regression model. 95% CI 95% confidence interval, AEH atypical endometrial hyperplasia, EEC endometrioid endometrial carcinoma, MM morular metaplasia, BMI body mass index, IR insulin resistance, DM diabetes mellitus, MS metabolic syndrome, CR complete response
Multivariate analyses showed that MM before treatment (HR 1.66, 95% CI 1.13–2.44, p = 0.011) was correlated with higher CR rate after adjusting for pathological diagnosis, MM, age at diagnosis, BMI, IR, hypertension, DM, MS, lesion size and regimen. MM during treatment (HR 0.62, 95% CI 0.44–0.87, p = 0.007) and lesion size > 2 cm (HR 0.62, 95% CI 0.50–0.76, p < 0.001) were independent risk factors for lower CR rate.
Factors associated with appearance of MM
To explore the possible risk factors associated with the appearance of MM in endometrial lesions, we first compared symptoms of chronic estrogen stimulation between MM group and non-MM group in Online Resource 1 and the results showed no significant difference. Second, logistic regression analysis was carried out in the three MM subgroups (Fig. 5). Multivariate logistic regression analysis showed that patients older than 30 years were less likely to have sustained MM (odds ratio 0.30, 95% CI 0.13–0.67, p = 0.003) after adjusting for pathological diagnosis, age at diagnosis, BMI, hypertension, DM, IR, MS and treatment regimens. No other risk factors were found to be correlated with appearance of MM either before or during fertility-preserving treatment.
Multi-variate subgroup analyses for factors associated with morular metaplasia according to logistic regression. BEF MM before treatment group, SUS sustained MM group, DUR MM during treatment group, 95% CI 95% confidence interval, AEH atypical endometrial hyperplasia, EEC endometrioid endometrial carcinoma, BMI body mass index, DM diabetes mellitus, IR insulin resistance, MS metabolic syndrome, MA megestrol acetate, MET metformin, other including levonorgestrel-releasing intrauterine system (LNG-IUS), ethinylestradiol cyproterone (Diane-35), or gonadotropin-releasing hormone analog (GnRH-a)
Discussion
In this study, we retrospectively analyzed the impact of MM on the outcome of fertility-preserving treatment in AEH and EEC patients. Our study showed that appearance of MM in different periods of progestin treatment had different clinical implications. Compared with the non-MM group, the BEF group was correlated with a higher CR rate and shorter therapeutic duration to achieve CR, while the DUR and SUS groups had poorer fertility-preserving treatment outcome.
The etiology of MM is not well understood. We did not find obvious risk factors correlated with the appearance of MM. Previous studies have shown that MM might be caused by chronic inflammation, such as chronic endometritis, submucosal leiomyoma, irradiation, or intrauterine devices [6, 7]. Other studies have reported that MM is associated with unopposed estrogen exposure or progestin treatment [18, 19]. Although the BEF, DUR and SUS groups had the same pathological characteristics, the different fertility-preserving treatment results implied that the etiology of MM in the BEF group differed from that in the DUR and SUS groups. MM that appeared only before treatment might be associated with prolonged estrogen exposure and thus sensitivity to progestin treatment. The SUS and DUR groups had similar fertility-preserving treatment results, which indicated similar etiology of MM in these two groups. The fact that more patients in the DUR group suffered from metabolic disorders such as hypertension, higher BMI and MS indicated that chronic inflammation caused by metabolic disorders might induce the appearance of MM, which could explain the poorer fertility-preserving treatment outcome in these two groups. It has been widely reported that chronic inflammation induced by metabolic disorders is negatively correlated with fertility-preserving treatment in AEH and EEC patients [12, 20, 21]. Further studies that test this hypothesis are warranted.
Our study had some clinical implications for fertility-preserving treatment in AEH and EEC patients. Appearance of MM before fertility-preserving treatment and its disappearance after treatment initiation implied a better treatment outcome, while sustained MM or appearance of MM only during fertility-preserving treatment implied poorer treatment results. These patients should be paid more attention, and treatment of complicated metabolic disorders might help improve fertility-preserving treatment. Furthermore, the fact that the SUS and DUR groups were younger than the non-MM group suggested that young AEH and EEC patients with metabolic disorders were more likely to have MM and develop resistance to progestin treatment.
The retrospective nature of our study was its main limitation and this study is subject to selection bias. A prospective study of both AEH and EEC patients might provide more information about the impact of MM on the result of fertility-preserving treatment in these patients. Additionally, molecular classification of endometrial lesion might help investigate the mechanism of appearance of MM. At last, there may be a small possibility that the morular metaplasia was not identified at the point of diagnosis.
In conclusion, our study showed that MM that appeared only before progestin treatment was positively correlated with the efficacy of fertility-preserving treatment in AEH and EEC patients, with a higher CR rate and shorter therapeutic duration to achieve CR. Patients with sustained MM or appearance of MM only during progestin treatment had poorer outcome of fertility-preserving treatment compared with patients in the non-MM group. Further studies are needed to confirm our findings and investigate the mechanisms involved.
References
Chinen K, Kamiyama K, Kinjo T, Arasaki A, Ihama Y, Hamada T, Iwamasa T (2004) Morules in endometrial carcinoma and benign endometrial lesions differ from squamous differentiation tissue and are not infected with human papillomavirus. J Clin Pathol 57(9):918–926. https://doi.org/10.1136/jcp.2004.017996
Lastra RR, McCluggage WG, Hedrick Ellenson L (2018) Benign diseases of the endometrium. In: Blaustein's pathology of the female genital tract. pp 1–63. https://doi.org/10.1007/978-1-4614-3165-7_7-2
Brachtel EF, Sanchez-Estevez C, Moreno-Bueno G, Prat J, Palacios J, Oliva E (2005) Distinct molecular alterations in complex endometrial hyperplasia (CEH) with and without immature squamous metaplasia (squamous morules). Am J Surg Pathol 29(10):1322–1329. https://doi.org/10.1097/01.pas.0000171001.87599.e2
Houghton O, Connolly LE, McCluggage WG (2008) Morules in endometrioid proliferations of the uterus and ovary consistently express the intestinal transcription factor CDX2. Histopathology 53(2):156–165. https://doi.org/10.1111/j.1365-2559.2008.03083.x
Hendrickson M, Kempson R (1980) Endometrial epithelial metaplasias: proliferations frequently misdiagnosed as adenocarcinoma. Report of 89 cases and proposed classification. Am J Surg Pathol 4(6):525–542
Nicolae A, Preda O, Nogales FF (2010) Endometrial metaplasias and reactive changes: a spectrum of altered differentiation. J Clin Pathol 64(2):97–106. https://doi.org/10.1136/jcp.2010.085555
Litta P, Codroma A, D’Agostino G, Breda E (2013) Morular endometrial metaplasia: review of the literature and proposal of the management. Eur J Gynaecol Oncol 34(3):243–247
Chiarelli S, Buritica C, Litta P, Ciani S, Guarch R, Nogales FF (2006) An immunohistochemical study of morules in endometrioid lesions of the female genital tract: CD10 is a characteristic marker of morular metaplasia. Clin Cancer Res 12(14 Pt 1):4251–4256. https://doi.org/10.1158/1078-0432.CCR-06-0398
Dutra FR (1959) Intraglandular morules of the endometrium. Am J Clin Pathol 31(1):60–65. https://doi.org/10.1093/ajcp/31.1.60
Koh W, Abu-Rustum N, Bean S, Bradley K, Campos S, Cho K, Chon H, Chu C, Cohn D (2019) Uterine Neoplasms, Version 1.2019, NCCN Clinical Practice Guidelines in Oncology
Yang B, Xu Y, Zhu Q, Xie L, Shan W, Ning C, Xie B, Shi Y, Luo X, Zhang H, Chen X (2019) Treatment efficiency of comprehensive hysteroscopic evaluation and lesion resection combined with progestin therapy in young women with endometrial atypical hyperplasia and endometrial cancer. Gynecol Oncol 153(1):55–62. https://doi.org/10.1016/j.ygyno.2019.01.014
Yang B, Xie L, Zhang H, Zhu Q, Du Y, Luo X, Chen X (2018) Insulin resistance and overweight prolonged fertility-sparing treatment duration in endometrial atypical hyperplasia patients. J Gynecol Oncol 29(3):e35. https://doi.org/10.3802/jgo.2018.29.e35
Smith KB, Smith MS (2016) Obesity statistics. Prim Care 43(1):121–135. https://doi.org/10.1016/j.pop.2015.10.001
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7):412–419. https://doi.org/10.1007/bf00280883
Shan W, Ning C, Luo X, Zhou Q, Gu C, Zhang Z, Chen X (2014) Hyperinsulinemia is associated with endometrial hyperplasia and disordered proliferative endometrium: a prospective cross-sectional study. Gynecol Oncol 132(3):606–610. https://doi.org/10.1016/j.ygyno.2014.01.004
Mishra GD, Pandeya N, Dobson AJ, Chung HF, Anderson D, Kuh D, Sandin S, Giles GG, Bruinsma F, Hayashi K, Lee JS, Mizunuma H, Cade JE, Burley V, Greenwood DC, Goodman A, Simonsen MK, Adami HO, Demakakos P, Weiderpass E (2017) Early menarche, nulliparity and the risk for premature and early natural menopause. Hum Reprod 32(3):679–686. https://doi.org/10.1093/humrep/dew350
Rotterdam EA-SPCWG (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 81(1):19–25. https://doi.org/10.1016/j.fertnstert.2003.10.004
Carlson JW, Mutter GL (2008) Endometrial intraepithelial neoplasia is associated with polyps and frequently has metaplastic change. Histopathology 53(3):325–332. https://doi.org/10.1111/j.1365-2559.2008.03104.x
Wheeler DT, Bristow RE, Kurman RJ (2007) Histologic alterations in endometrial hyperplasia and well-differentiated carcinoma treated with progestins. Am J Surg Pathol 31(7):988–998. https://doi.org/10.1097/PAS.0b013e31802d68ce
Zhou R, Yang Y, Lu Q, Wang J, Miao Y, Wang S, Wang Z, Zhao C, Wei L (2015) Prognostic factors of oncological and reproductive outcomes in fertility-sparing treatment of complex atypical hyperplasia and low-grade endometrial cancer using oral progestin in Chinese patients. Gynecol Oncol 139(3):424–428. https://doi.org/10.1016/j.ygyno.2015.09.078
Chen M, Jin Y, Li Y, Bi Y, Shan Y, Pan L (2016) Oncologic and reproductive outcomes after fertility-sparing management with oral progestin for women with complex endometrial hyperplasia and endometrial cancer. Int J Gynaecol Obstet 132(1):34–38. https://doi.org/10.1016/j.ijgo.2015.06.046
Acknowledgements
We are grateful for the data work of Bingyi Yang, Yue Shi, and Lulu Wang.
Funding
This work was funded by the National Key Technology R&D Program of China (Grant number 2019YFC1005203), the Science and Technology Commission of Shanghai Municipality (Grant number 18ZR1405300), and the Shanghai Sailing Program (Grant number 19YF1404200).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection was performed by PW, QL, JG and WS. Data analysis was performed by PW and JG. The first draft of the manuscript was written by PW and QL and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval
The study was approved by the Medical Ethics Committee of Obstetrics and Gynecology Hospital of Fudan University.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, P., Lv, Q., Guan, J. et al. Clinical implications of morular metaplasia in fertility-preserving treatment for atypical endometrial hyperplasia and early endometrial carcinoma patients. Arch Gynecol Obstet 306, 1135–1146 (2022). https://doi.org/10.1007/s00404-021-06382-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-021-06382-3