Abstract
Purpose
Hysterectomy has been associated with increased risk for developing stress urinary incontinence (SUI) and having a SUI operation. We examined the long-term rate of SUI operations after hysterectomy and associated risk factors.
Methods
We followed up 5000 women without prior urinary incontinence (UI) who had a hysterectomy in a prospective FINHYST 2006 cohort study until the end of 2016 through a national health register. The main outcome was SUI operations, and secondary outcomes were outpatient visits for UI, and their association of preoperative patient and operation factors.
Results
During the median follow-up time of 10.6 years (IQR 10.3–10.8), 111 (2.2%) women had a SUI operation and 241 (4.8%) had an outpatient visit for UI. The SUI operation rate was higher after vaginal hysterectomy and laparoscopic hysterectomy (n = 71 and 28, 3.3% and 1.8%, respectively) compared to abdominal hysterectomy (n = 11, 0.8%). In a multivariate risk analysis by Cox regression, the association with vaginal hysterectomy and SUI operation remained significant when adjusted for vaginal deliveries, preceding pelvic organ prolapse (POP), uterus size, age and BMI (HR 2.4, 95% CI 1.1–5.3). Preceding POP, three or more deliveries and laparoscopic hysterectomy were significantly associated with UI visits but not with SUI operations.
Conclusion
After hysterectomy, 2.2% of women underwent operative treatment for SUI. The number of SUI operations was more than double after vaginal hysterectomy compared to abdominal hysterectomy, but preceding POP explained this added risk partially. Preceding POP and three or more vaginal deliveries were independently associated with UI visits after hysterectomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hysterectomy is a commonly used procedure to treat benign conditions such as abnormal uterine bleeding, pelvic organ prolapse (POP), and uterine fibroids. In the US alone, 433,621 inpatient hysterectomies were performed in 2010 [1]. The hysterectomy approach is decided mainly based on the indication and size of the uterus, but other patient-related factors and preferences of the gynecologic surgeon also affect the choice. Cochrane review recommends vaginal hysterectomy (VH) whenever possible, and in 2006 in Finland, VH was the most common approach, followed by laparoscopic hysterectomy (LH), which has largely replaced the abdominal hysterectomy (AH) [2, 3].
The way hysterectomy affects the pelvic anatomy and causes surgical trauma to the nerve supply is thought to cause the doubled risk of developing stress urinary incontinence (SUI) [4], defined as involuntary leakage of urine on effort or exertion [5]. Similarly, other factors that cause pressure or trauma to the pelvic floor, such as obesity, pregnancies and vaginal deliveries increase the risk of SUI [6]. However, the role of indication for hysterectomy, concomitant prolapse, concomitant surgery or the experience of the surgeon on the risk of SUI after hysterectomy remains unclear. The independent effect of the selected hysterectomy approach has also been hard to establish due to confounding factors [4].
In this prospective cohort study, we follow-up 5000 women for 10 years after a hysterectomy performed for a benign indication in 2006 and report the number of SUI operations and outpatient visits for SUI and other urinary incontinence (UI). We also assess the association of patient and operation factors with the long-term risk for SUI operations and UI visits.
Materials and methods
A prospective national FINHYST 2006 study of 5279 women who underwent a hysterectomy for a benign indication was conducted in 53 Finnish hospitals in 2006, and it included 79% of all hysterectomies for benign indications in Finland that year. All women provided written informed consent before the study, and they also gave permission for further analyses. Data were collected in 2006 by surveys to gynecological surgeons and their patients as described in detail previously [2].
The follow-up data were extracted from the Care Register for Health Care (Care Register) maintained by the Finnish Institute for Health and Welfare. The Care Register contains diagnoses and operation codes for all in- and outpatient visits in every private and public hospital in Finland. The validity of the Care Register with respect to different medical conditions has been evaluated as satisfactory to very good in numerous studies, and a few validation studies have been published in the field of gynecology [7]. From the Care Register, we identified the sample women’s visits for urinary incontinence (ICD-10 code N39.3 for SUI, and N39.4 for other UI), visits for pelvic organ prolapse (ICD-10 code N81* and NCSP code LEF*) and SUI operations (NCSP codes LEG*, KDG*, KDV20 and KDV22) from 1996 to the end of 2016.
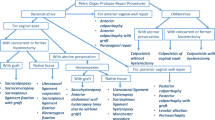
The hysterectomy approaches were classified as vaginal, abdominal, and laparoscopic, and both total and subtotal hysterectomies were included. In the event of conversion, hysterectomy approach was classified according to the final operation method. We excluded women (n = 279) who had a concomitant UI operation recorded in the FINHYST 2006 data or visits with UI diagnosis or SUI operation codes (ICD-10 codes N39.3 and N39.4, NCSP codes LEG*, KDG*, KDV20, and KDV22) before or concomitant with the hysterectomy in the Care Register producing a final sample size of 5000 (Fig. 1).
Women were considered to have a preceding POP if the FINHYST 2006 survey data reported POP as the main indication or a concomitant POP operation, or if there was a hospital visit recorded in the Care Register before the hysterectomy with a diagnosis code for POP (ICD-10 N81*) or POP operation (NCSP code LEF*). As the indication, we used the single main preoperative indication selected by the gynecologic surgeon in the preoperative survey (myoma, menorrhagia, dysmenorrhea, endometriosis, uterine prolapse, adnexal mass or other). Other patient and operation characteristics were defined as described previously [2].
Our main outcome was SUI operation 60 days or more after the hysterectomy, which we defined as a visit with a SUI operation code (NCSP codes LEG*, KDG*, KDV20 and KDV22) in the Care Register. Our secondary outcome was UI visits 60 days or more after hysterectomy, which we defined as a visit with a UI diagnosis code (ICD-10 N39.3 for SUI, and N39.4 for other UI) in the Care Register. Only the first operation for SUI or visit for UI was reported for each woman. We defined UI visits that also had a diagnosis code for POP (ICD-10 N81*) as UI visits with concomitant POP.
In the risk factor analysis, we first conducted a univariate analysis for hysterectomy approach, age, BMI, preceding abdominal operations (including caesarean sections, laparoscopies, and laparotomies), preceding POP, parity, vaginal deliveries, indication, uterus size, concomitant operations, intraoperative complications, experience of the surgeon, and hospital type. We tested the variables that were found significant in the univariate analysis in a multivariate Cox regression analysis adjusted with each other. We conducted a similar risk analysis including only women without a preceding POP (n = 3452). For visualization, we made a Kaplan–Meier curves for cumulative survival without a SUI operation according to the hysterectomy method and preceding POP. When comparing more than two groups, we used one-way ANOVA to calculate the p value. A significance level of p < 0.05 was used unless stated otherwise. IBM SPSS Statistics 25 was used for statistical analysis.
The study protocol was approved by the Ethical Committee of the Helsinki and Uusimaa Hospital District (Dnro 457/E8/04 and 343/13/03/03/2015) and was registered in the Clinical Trials (NCT00744172). The Finnish Institute for Health and Welfare of Finland authorized the use of the data from the Care Register (THL/986/5.05.00/2018).
Results
Of the 5000 hysterectomies, VH was most common, followed by LH and AH (Table 1). Subtotal procedures were rare: in AH 85 (6.5%) and in LH 3 (0.2%). Fibroids were the most common indication of all hysterectomies, followed by POP in the VH group. Concomitant surgery was performed in 2743 (55%) hysterectomies. Bilateral adnexal removal was performed for 36% AH and 32% in LH groups, while concomitant POP procedures were performed for 54% in the VH group.
During the follow-up time of 10.6 median years (IQR 10.3–10.8), 111 (2.2%) women had a SUI operation (Table 2). The rate of SUI operations was significantly higher after VH (n = 71, 3.3%) than after AH and LH (n = 11, 0.8% and n = 28, 1.8%, respectively, p < 0.05). The mid-urethral sling was the predominant operation type (97%), and the transobturator route was slightly more frequently used than retropubic route (53% and 44%, respectively). The operations were mostly performed due to SUI (n = 96, 87%). Of all women, 241 (4.8%) had a hospital visit for urinary incontinence, of which 142 (59%) were for SUI.
In the univariate analysis, the SUI operations and UI visits were significantly associated with LH and VH when AH was used as the reference (Table 3). Preceding POP and POP as the main indication were equally associated with SUI operation and UI visit, while other indications were insignificant. In a sub-analysis including only patients with a preceding POP (n = 1548), a concomitant POP operation did not affect the risk for SUI operation or UI visit (HR 1.0 and 0.9, 95% CI 0.5–2.0 and 0.6–1.3, respectively). Age, BMI, experience or status of the surgeon, hospital type, preceding abdominal operations, concomitant adnexal removal, any concomitant operation, and perioperative complications (any, minor, or major) were found statistically insignificant in univariate analysis (p > 0.2).
In multivariate analysis, only vaginal hysterectomy was associated with a significantly higher risk for SUI operations (HR 2.4, 95% CI 1.1–5.3, see Table 4). Patients with a higher number of vaginal deliveries, smaller uterus size and preceding POP did have more SUI operations, but this difference did not reach statistical significance. As to UI visits, a significant association was found with preceding POP (HR 2.0, 95% CI 1.4–2.9), three or more vaginal deliveries (HR 1.7, 95% CI 1.1–2.5), and LH (HR 1.6, 95% CI 1.0–2.6). Preceding POP was significantly associated with other UI visits but not with SUI visits (HR 3.1 and 1.5, 95% CI 1.8–5.6 and 0.9–2.3, respectively).
In the univariate sub-analysis including only women without preceding POP, LH and VH as the hysterectomy method were found significantly associated with both SUI operations (HR 2.0 and 3.4, 95% CI 1.0 and 4.1, respectively) and UI visits (HR 1.5 and 1.6, 95% CI 1.0–2.4 and 1.0–2.6, respectively). Uterus larger than 500 g was found negatively associated with SUI operations and visits (HR 0.2 and 0.4, 95% CI 0.1–0.9 and 0.2–0.9, respectively). Three or more vaginal deliveries were found significantly associated with UI visits but not with SUI operations (HR 1.8 and 1.4, 95% CI 1.1–3.0 and 0.7–3.1) when no vaginal deliveries was used as the reference. One to two vaginal deliveries, age, BMI, preceding abdominal operations, vaginal deliveries, main indication, concomitant procedures, experience and status of the surgeon and hospital type were found insignificant. In the multivariate sub-analysis including only women without preceding POP, VH remained significantly associated with SUI operations (HR 2.6, 95% CI 1.2–5.7) and three or more vaginal deliveries with UI visits (HR 1.8, 95% CI 1.1–3.0). See details of the multivariate analysis in Table 4.
Kaplan–Meier curves of survival without SUI operation (Figs. 2, 3) show that SUI operations occurred throughout the follow-up time. However, most UI visits (59%) and SUI operations (57%) occurred within the first 5 years after the hysterectomy. The difference in the cumulative survival between the different hysterectomy approaches and women with or without preceding POP lasts throughout the follow-up time. The median time from hysterectomy to SUI operations and UI visits did not differ significantly between the different hysterectomy approaches (p = 0.3). The median time between hysterectomy and UI visit was significantly shorter for women with preceding POP compared to women without preceding POP (median time in years 2.6 and 4.3, IQR 0.6–6.5 and 1.7–6.6, respectively, p < 0.05).
Discussion
In this prospective cohort study of 5000 hysterectomies on women without prior UI, we assessed the risk for SUI operation after hysterectomy. The long follow-up time of over 10 years ensured that our study most likely could record all new UI cases since our data are similar to the previous study by Altman et al., with the risk being highest up to 5 years after hysterectomy [4]. Even though only 2.2% of women had a SUI operation 10 years after hysterectomy, the rate of SUI operations was twice higher than expected based on national incidence of SUI operations in Finland in 2009 [8]. The rate of SUI operations was approximately half of the rate of UI visits (2.2% vs 4.8%), which indicates that some of the women seeking help managed with conservative treatments.
We found the risk for SUI operation to be over twice higher after VH than AH even after adjusting for other risk factors and when only women without preceding POP were included in the analysis. This is in line with a previous large cohort study in Sweden [9]. However, because the choice of hysterectomy method was not randomized, it is possible that the women in the VH group had favorable anatomy for VH such as mobile uterus or latent POP.
Differences of re-establishing apical support between hysterectomy approaches may also explain the added risk for SUI operations and UI visits after LH compared to AH. At the time of the sample hysterectomies, uterosacral ligament fixation was not used in LH, which may explain the significantly higher risk for UI visits after LH compared to AH. Re-establishing apical support at hysterectomy is recommended to prevent post-hysterectomy POP [10], but its effect on de novo UI after hysterectomy is not established, and in the future it will be interesting to see whether laparoscopic suturing of the vaginal cuff with uterosacral ligament fixation will decrease the risk of UI. However, because uterosacral ligament fixation is used in VH, the differences in building apical support does not explain the risk associated with VH. It is possible that at least some women in VH group had latent POP, and dysfunction of pelvic floor resulted also in UI. Also, most women with uterus larger than 500 g had AH. It is possible that a uterus too large to prolapse may protect structures from stretching down, and thus, decrease the risk for UI after AH. Even though the protective effect of a uterus larger than 500 g was no longer significant in our multivariate analysis, previously, a uterus larger than 500 g has been associated with a lower risk for UI after hysterectomy [11].
Preceding POP significantly increased the risk for UI visit but not for SUI operation when adjusted for other risk factors. This result is supported by a previous study that reported an unadjusted HR of 1.7 for SUI operation after hysterectomy with POP as indication [4]. In our data, preceding POP had a stronger association with visits for other UI than with visits for SUI. It is likely that many of these women suffered from urge dominant UI, which is treated conservatively. Therefore, this finding could explain why the risk for SUI operation remained insignificant. Interestingly, women with a concomitant POP operation had a similar rate of SUI operations and UI visits than women with a preceding POP without a concomitant POP operation. Association between SUI and POP has been noted previously, and after a POP operation without hysterectomy, up to 39% of women without SUI and 52% of women with occult SUI preoperatively present with objective SUI [12]. These data reflect that both SUI and POP are a result of pelvic floor dysfunction and a complex collection of interconnected symptoms.
Even though bilateral adnexal removal can also be performed in VH, it is often easier to perform in AH or LH. Unsurprisingly, only 5% of hysterectomies in our sample with a concomitant bilateral adnexal removal were performed in VH. This uneven distribution between hysterectomy approaches explains why bilateral adnexal removal was found significantly associated with SUI operations and UI visits only in the univariate analysis but not in the multivariate analysis.
The strengths of this study include a large prospective sample of 5000 women, unselected population-based design that represents real-life clinical setting, long follow-up time of 10.6 years in median and clear end-point data from a reliable register. The detailed patient and operation characteristics data collected preoperatively allowed us to control confounding factors such as BMI, age, preceding POP, vaginal deliveries, uterus size, and concomitant deliveries. Furthermore, being able to distinguish SUI visits from visits for other UI allowed us to analyze risk factors for pure SUI without confounding other types of UI.
As a limitation, we recognize that we were not able to identify UI in women who did not seek treatment or were not referred to specialized health care. However, we believe that the operations and visits represent well bothersome UI due to good access to healthcare in Finland. Using SUI operations and UI visits as endpoints also resulted in a low number of end-point events and lower statistical power despite the large sample size. Because the hysterectomy method was not chosen randomly, there were many demographic differences between AH, LH and VH groups. To overcome this selection bias, we used multivariable analysis and performed sub-group analysis when needed, but we acknowledge that not all potentially significant confounders such as uterus mobility were captured in the data. Even though BMI is a known risk factor for SUI, in our sample it was not significantly associated with SUI operations or visits. This result may be biased, because obesity is a relative contraindication for surgical treatment of UI as well as hysterectomy. Also, even though we had detailed information collected at the time of the hysterectomy, the profession, menopausal status, comorbidities and home medications were not included in our data.
To conclude, during the follow-up time of ten years, 2.2% of women had a SUI operation after hysterectomy. The association of VH and SUI operations remained significant even after adjusting for other risk factors and in a sub-analysis including only women without preceding POP. Preceding POP was significantly associated with UI visits, and this association was stronger with other UI visits than SUI visits. Also, three or more vaginal deliveries and LH were significantly associated with UI visits.
Availability of data and material
The data and material are not available.
Code availability
IBM SPSS Statistics 25 was used for statistical analysis.
References
Wright JD, Herzog TJ, Tsui J, Ananth CV, Lewin SN, Lu YS et al (2013) Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol 122(2 Pt 1):233–241
Brummer TH, Jalkanen J, Fraser J, Heikkinen AM, Kauko M, Makinen J et al (2009) FINHYST 2006-national prospective 1-year survey of 5279 hysterectomies. Hum Reprod 24(10):2515–2522
Aarts JW, Nieboer TE, Johnson N, Tavender E, Garry R, Mol BW et al (2015) Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev 8:CD003677
Altman D, Granath F, Cnattingius S, Falconer C (2007) Hysterectomy and risk of stress-urinary-incontinence surgery: nationwide cohort study. Lancet 370(9597):1494–1499
Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U et al (2003) The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology 61(1):37–49
Danforth KN, Townsend MK, Lifford K, Curhan GC, Resnick NM, Grodstein F (2006) Risk factors for urinary incontinence among middle-aged women. Am J Obstet Gynecol 194(2):339–345
Sund R (2012) Quality of the Finnish Hospital Discharge Register: a systematic review. Scand J Public Health 40(6):505–515
Kurkijarvi K, Aaltonen R, Gissler M, Makinen J (2016) Surgery for stress urinary incontinence in Finland 1987–2009. Int Urogynecol J 27(7):1021–1027
Forsgren C, Lundholm C, Johansson AL, Cnattingius S, Zetterstrom J, Altman D (2012) Vaginal hysterectomy and risk of pelvic organ prolapse and stress urinary incontinence surgery. Int Urogynecol J 23(1):43–48
AAGL Advancing Minimally Invasive Gynecology Worldwide (2014) AAGL practice report: practice guidelines on the prevention of apical prolapse at the time of benign hysterectomy. J Minim Invasive Gynecol 21(5):715–722
Bohlin KS, Ankardal M, Lindkvist H, Milsom I (2017) Factors influencing the incidence and remission of urinary incontinence after hysterectomy. Am J Obstet Gynecol 216(1):53.e1-53.e9
van der Ploeg JM, van der Steen A, Oude Rengerink K, van der Vaart CH, Roovers JP (2014) Prolapse surgery with or without stress incontinence surgery for pelvic organ prolapse: a systematic review and meta-analysis of randomised trials. BJOG 121(5):537–547
Acknowledgements
Tulokas acknowledges receiving a research grant from a non-industry foundation Viipurin Tuberkuloosisäätiö while conducting this study.
Funding
Open Access funding provided by University of Helsinki including Helsinki University Central Hospital. Tulokas declares receiving a research grant from a non-industry foundation Viipurin Tuberkuloosisäätiö while conducting this study.
Author information
Authors and Affiliations
Contributions
ST: protocol and project development, data collection and management, data analysis, manuscript writing and editing. MM: protocol and project development, manuscript writing and editing. PH: protocol and project development, manuscript writing and editing. TB: data collection and management, manuscript writing and editing. JJ: data collection and management, manuscript writing and editing. TK: data collection and management, manuscript writing and editing. JM: data collection and management, manuscript writing and editing. JS: data collection and management, manuscript writing and editing. ET: data collection and management, manuscript writing and editing. PR-S: protocol and project development, manuscript writing and editing.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest or competing interests.
Ethics approval
The study protocol was approved by the Ethical Committee of the Helsinki and Uusimaa Hospital District (Dnro 457/E8/04 and 343/13/03/03/2015) and was registered in the Clinical Trials (NCT00744172). The Finnish Institute for Health and Welfare of Finland authorized the use of the data from the Care Register (THL/986/5.05.00/2018).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tulokas, S., Mentula, M., Härkki, P. et al. Stress urinary incontinence after hysterectomy: a 10-year national follow-up study. Arch Gynecol Obstet 305, 1089–1097 (2022). https://doi.org/10.1007/s00404-021-06378-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-021-06378-z