Abstract
Introduction
The pathogen-associated molecular patterns and the danger-associated molecular patterns are possibly responsible for the activation of the inflammatory process in endometriosis through the activation of toll-like receptors (TLRs).
Objective
The aim of this systematic review was to critically analyze the findings of published articles on TLRs in endometriosis.
Methods
The keywords used were “endometriosis” and “toll-like” and the search was performed in Pubmed, Scielo and Lilacs databases. This study followed the PRISMA guidelines and the risk of bias of articles was conducted by Newcastle–Ottawa scale (NOS).
Results
Overall, the studies analyzed in this review point toward an increased expression of TLRs two, four and nine in women with endometriosis. Among all TLRs, TLR4 was the most cited receptor.
Conclusion
Despite the evidence demonstrating elevated TLR levels in endometriosis, the relationship with the disease is still unclear and needs to be clarified in further studies about innate immune response.



Similar content being viewed by others
References
Parasar P, Ozcan P, Terry KL (2017) Endometriosis: epidemiology, diagnosis and clinical management. Curr Obstet Gynecol Rep. https://doi.org/10.1007/s13669-017-0187-1
Nisolle M, Donnez J (1997) Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil Steril. https://doi.org/10.1016/S0015-0282(97)00191-X
Santulli P, Tran C, Gayet V et al (2018) Oligo-anovulation is not a rarer feature in women with documented endometriosis. Fertil Steril. https://doi.org/10.1016/j.fertnstert.2018.06.012
Khan KN, Fujishita A, Masumoto H et al (2016) Molecular detection of intrauterine microbial colonization in women with endometriosis. Eur J Obstet Gynecol Reprod Biol. https://doi.org/10.1016/j.ejogrb.2016.01.040
Klemmt PA, Starzinski-Powitz A (2017) Molecular and cellular pathogenesis of endometriosis. Curr Women’s Health Rev. https://doi.org/10.2174/1573404813666170306163448
Gou Y, Li X, Li P et al (2019) Estrogen receptor β upregulates CCL2 via NF-κB signaling in endometriotic stromal cells and recruits macrophages to promote the pathogenesis of endometriosis. Hum Reprod. https://doi.org/10.1093/humrep/dez019
Huhtinen K, DesaiStah̊le RM et al (2012) Endometrial and endometriotic concentrations of estrone and estradiol are determined by local metabolism rather than circulating levels. J Clin Endocrinol Metab. https://doi.org/10.1210/jc.2012-1154
Scutiero G, Iannone P, Bernardi G, et al (2017) Oxidative stress and endometriosis: a systematic review of the literature. Oxid Med Cell Longev 2017:1–7
Podgaec S, Dias Junior JA, Chapron C et al (2010) Th1 and Th2 immune responses related to pelvic endometriosis. Rev Assoc Med Bras. https://doi.org/10.1590/s0104-42302010000100022
Podgaec S, Rizzo LV, Fernandes LFC et al (2012) CD4+CD25high FoxP3+cells increased in the peritoneal fluid of patients with endometriosis. Am J Reprod Immunol. https://doi.org/10.1111/j.1600-0897.2012.01173.x
Sourial S, Tempest N, Hapangama DK (2014) Theories on the pathogenesis of endometriosis. Int J Reprod Med. https://doi.org/10.1155/2014/179515
Bellelis P, Frediani Barbeiro D, Gueuvoghlanian-Silva BY et al (2019) Interleukin-15 and interleukin-7 are the major cytokines to maintain endometriosis. Gynecol Obstet Invest. https://doi.org/10.1159/000496607
Dull AM, Moga MA, Dimienescu OG et al (2019) Therapeutic approaches of resveratrol on endometriosis via anti-inflammatory and anti-angiogenic pathways. Molecules 24:667
Khan KN, Fujishita A, Hiraki K et al (2018) Bacterial contamination hypothesis: a new concept in endometriosis. Reprod Med Biol 17:125–133
Trinchieri G, Sher A (2007) Cooperation of toll-like receptor signals in innate immune defence. Nat Rev Immunol 7:179–190
Kawai T, Akira S (2009) The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol 21:317–337
Lamkanfi M, Dixit VM (2014) Mechanisms and functions of inflammasomes. Cell 157:1013–1022
Broz P, Dixit VM (2016) Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol 16:407–420
Sharma D, Kanneganti TD (2016) The cell biology of inflammasomes: mechanisms of inflammasome activation and regulation. J Cell Biol 213:617–629
Bullon P, Navarro JM (2017) Inflammasome as a key pathogenic mechanism in endometriosis. Curr Drug Targets. https://doi.org/10.2174/1389450117666160709013850
Webster SJ, Goodall JC (2018) New concepts in chlamydia induced inflammasome responses. Microbes Infect. https://doi.org/10.1016/j.micinf.2017.11.011
Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124:783–801
Kobayashi H, Higashiura Y, Shigetomi H, Kajihara H (2014) Pathogenesis of endometriosis: the role of initial infection and subsequent sterile inflammation (review). Mol Med Rep. https://doi.org/10.3892/mmr.2013.1755
Khan KN, Kitajima M, Hiraki K et al (2009) Toll-like receptors in innate immunity: role of bacterial endotoxin and toll-like receptor 4 in endometrium and endometriosis. Gynecol Obstet Invest 68:40–52
Khan KN, Kitajima M, Inoue T et al (2013) Additive effects of inflammation and stress reaction on toll-like receptor 4-mediated growth of endometriotic stromal cells. Hum Reprod. https://doi.org/10.1093/humrep/det280
Moher D, Liberati A, Tetzlaff J, Altman DG (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. https://doi.org/10.1016/j.ijsu.2010.02.007
Wells GA, Shea B, O’connell D et al (2014) The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hosp Res Inst http://www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf
Malvezzi H, Marengo EB, Podgaec S, Piccinato CDA (2020) Endometriosis: current challenges in modeling a multifactorial disease of unknown etiology. J Transl Med 18:1–21
Khan KN, Kitajima M, Imamura T et al (2008) Toll-like receptor 4-mediated growth of endometriosis by human heat-shock protein 70. Hum Reprod. https://doi.org/10.1093/humrep/den195
Khan KN, Kitajima M, Hiraki K et al (2010) Escherichia coli contamination of menstrual blood and effect of bacterial endotoxin on endometriosis. Fertil Steril 94:2860-2863.e3. https://doi.org/10.1016/j.fertnstert.2010.04.053
Luo XZ, ZhouTao WJY et al (2015) TLR4 activation promotes the secretion of IL-8 which enhances the invasion and proliferation of endometrial stromal cells in an autocrine manner via the FAK signal pathway. Am J Reprod Immunol. https://doi.org/10.1111/aji.12425
Allhorn S, Böing C, Koch AA et al (2008) TLR3 and TLR4 expression in healthy and diseased human endometrium. Reprod Biol Endocrinol. https://doi.org/10.1186/1477-7827-6-40
Hayashi C, Chishima F, Sugitani M et al (2013) Relationship between toll-like receptor-4 and mPGES-1 gene expression in local lesions of endometriosis patients. Am J Reprod Immunol. https://doi.org/10.1111/aji.12056
Yeo SG, Won YS, Lee HY et al (2013) Increased expression of pattern recognition receptors and nitric oxide synthase in patients with endometriosis. Int J Med Sci. https://doi.org/10.7150/ijms.5169
Sobstyl M, Niedźwiedzka-Rystwej P, Grywalska E et al (2020) Toll-like receptor 2 expression as a new hallmark of advanced endometriosis. Cells. https://doi.org/10.3390/cells9081813
Hernandes C, Gueuvoghlanian-Silva BY, Monnaka VU et al (2020) Regulatory T cells isolated from endometriotic peritoneal fluid express a different number of toll-like receptors. Einstein (Sao Paulo). https://doi.org/10.31744/einstein_journal/2020AO5294
Kajihara H, Yamada Y, Kanayama S et al (2011) New insights into the pathophysiology of endometriosis: from chronic inflammation to danger signal. Gynecol Endocrinol 27:73–79
Miyake K (2007) Innate immune sensing of pathogens and danger signals by cell surface Toll-like receptors. Semin Immunol 19:3–10
González-Ramos R, Donnez J, Defrère S et al (2007) Nuclear factor-kappa B is constitutively activated in peritoneal endometriosis. Mol Hum Reprod. https://doi.org/10.1093/molehr/gam033
Aggarwal BB (2004) Nuclear factor-κB: the enemy within. Cancer Cell 6:203–208
Park M, Hong J (2016) Roles of NF-κB in cancer and inflammatory diseases and their therapeutic approaches. Cells. https://doi.org/10.3390/cells5020015
Lingappan K (2018) NF-κB in oxidative stress. Curr Opin Toxicol 7:81–86
Lebovic DI, Chao VA, Martini J-F, Taylor RN (2001) IL-1β induction of RANTES (regulated upon activation, normal T cell expressed and secreted) chemokine gene expression in endometriotic stromal cells depends on a nuclear factor-κB site in the proximal promoter. J Clin Endocrinol Metab 86:4759–4764. https://doi.org/10.1210/jcem.86.10.7890
Sakamoto Y, Harada T, Horie S et al (2003) Tumor necrosis factor-α-induced interleukin-8 (IL-8) expression in endometriotic stromal cells, probably through nuclear factor-κB activation: gonadotropin-releasing hormone agonist treatment reduced IL-8 expression. J Clin Endocrinol Metab. https://doi.org/10.1210/jc.2002-020666
Iba Y, Harada T, Horie S et al (2004) Lipopolysaccharide-promoted proliferation of endometriotic stromal cells via induction of tumor necrosis factor α and interleukin-8 expression. Fertil Steril. https://doi.org/10.1016/j.fertnstert.2004.04.038
Yamauchi N, Harada T, Taniguchi F et al (2004) Tumor necrosis factor-α induced the release of interleukin-6 from endometriotic stromal cells by the nuclear factor-κB and mitogen-activated protein kinase pathways. Fertil Steril. https://doi.org/10.1016/j.fertnstert.2004.02.134
Horie S, Harada T, Mitsunari M et al (2005) Progesterone and progestational compounds attenuate tumor necrosis factor alpha-induced interleukin-8 production via nuclear factor kappa B inactivation in endometriotic stromal cells. Fertil Steril. https://doi.org/10.1016/j.fertnstert.2004.11.042
González-Ramos R, Van Langendonckt A, Defrre S et al (2010) Involvement of the nuclear factor-κB pathway in the pathogenesis of endometriosis. Fertil Steril. https://doi.org/10.1016/j.fertnstert.2010.01.013
González-Ramos R, Defrère S, Devoto L (2012) Nuclear factor-kappa B: a main regulator of inflammation and cell survival in endometriosis pathophysiology. Fertil Steril 98:520–528
Sikora J, Mielczarek-Palacz A, Kondera-Anasz Z (2012) Imbalance in cytokines from interleukin-1 family—role in pathogenesis of endometriosis. Am J Reprod Immunol. https://doi.org/10.1111/j.1600-0897.2012.01147.x
Akoum A, Al-Akoum M, Lemay A et al (2008) Imbalance in the peritoneal levels of interleukin 1 and its decoy inhibitory receptor type II in endometriosis women with infertility and pelvic pain. Fertil Steril. https://doi.org/10.1016/j.fertnstert.2007.06.019
Ejzenberg D (2007) Avaliação das concentrações das interleucinas 1-beta e 6 e da proteína amilóide A, no líquido peritoneal e no soro de pacientes com endometriose pélvica. Universidade de São Paulo 2007:1–119
Gueuvoghlanian-Silva BY, Bellelis P, Barbeiro DF et al (2018) Treg and NK cells related cytokines are associated with deep rectosigmoid endometriosis and clinical symptoms related to the disease. J Reprod Immunol. https://doi.org/10.1016/j.jri.2018.02.003
Funding
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Author information
Authors and Affiliations
Contributions
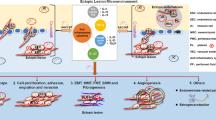
BCA and SP contributed to conception and design of the systematic review. BCA conducted the literature search, title/abstract review, abstracted. BCA and SP conducted the quality-assessed the included studies. FM prepared the Fig. 3, reviewed, and edited the manuscript. BCA and SP contributed to writing and revising the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This is an observational study. The Research Ethics Committee at Hospital Israelita Albert Einstein–CEP/Einstein, has confirmed that no ethical approval is required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Azevedo, B.C., Mansur, F. & Podgaec, S. A systematic review of toll-like receptors in endometriosis. Arch Gynecol Obstet 304, 309–316 (2021). https://doi.org/10.1007/s00404-021-06075-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-021-06075-x