Abstract
Purpose
The commonest surgical procedure for women is cesarean delivery. Postpartum hemorrhage and intra-operative blood during cesarean delivery is a major concern to all obstetricians. This study was conducted to assess the efficacy of the adjuvant use of misoprostol and oxytocin in decreasing intra-operative blood loss in cesarean delivery.
Methods
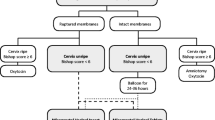
This was a double-blinded randomized clinical trial including 636 term pregnant woman scheduled for cesarean section at Ain Shams University Maternity Hospital, Cairo, Egypt, between February 2013 and February 2014. Participants received either 400-μg misoprostol rectally or sublingually or placebo before cesarean section together with 5-IU oxytocin IV. The main outcome measure was intra-operative blood loss. Difference between the three groups was analyzed using one-way ANOVA test (for numeric variables) and Chi-square test (for categorical variables). P < 0.05 was considered statistically significant.
Results
Intra-operative blood loss was higher in patients who did not receive misoprostol (Placebo Group) (295–1075 ml, 641.7 ± 135.7) than those who received it, regardless the route of administration, rectal (135–830 ml, 457.5 ± 140.7; P < 0.001), and sublingual (135–680 ml, 357.8 ± 129.7; P < 0.001). In addition, sublingual route was associated with significantly lower estimated intra-operative blood loss compared to rectal administration (P < 0.001).
Conclusions
Misoprostol with oxytocin is an effective drug-combination for decreasing intra-operative blood loss during cesarian section with clinical superiority to sublingual over rectal route.

Similar content being viewed by others
Change history
12 March 2024
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s00404-024-07428-y
References
Ananth CV, Vintzileos AM (2011) Trends in cesarean delivery at preterm gestation and association with perinatal mortality. Am J Obstet Gynecol 204:505–508
Getahun D, Strickland D, Lawrence JM, Fassett MJ, Koebnick C, Jacobsen SJ (2009) Racial and ethnic disparities in the trends in primary cesarean delivery based on indications. Am J Obstet Gynecol 201:422–427
Khawaja M, Choueiry N, Jurdi R (2009) Hospital-based caesarean section in the Arab region: an overview. East Mediterr Health J 15:458–469
ACOG Practice Bulletin (2006) Clinical management guidelines for obstetrician-gynecologists number 76, October 2006: postpartum hemorrhage. Obstet Gynecol 108:1039–1047
Langenbach C (2006) Misoprostol in preventing postpartum hemorrhage: a meta-analysis. Int J Gynecol Obstet 92:10–18
Munn MB, Owen J, Vincent R, Wakefield M, Chestnut DH, Hauth JC (2001) Comparison of two oxytocin regimens to prevent uterine atony at cesarean delivery: a randomized controlled trial. Obstet Gynecol 98:386–390
World Health Organization (2012) WHO recommendations for the prevention and treatment of postpartum hemorrhage. World Health Organization, Geneva
Gulmezoglu AM, Forna F, Villar J, Hofmeyr GJ (2007) Prostaglandins for preventing postpartum haemorrhage. Cochrane Database Syst Rev 8(3):494
Conde-Agudelo A, Nieto A, Rosas-Bermudez A, Romero R (2013) Misoprostol to reduce intraoperative and postoperative hemorrhage during cesarean delivery: a systematic review and metaanalysis. Am J Obstet Gynecol 209:40
Soltan MH, El-Gendi E, Imam HH, Fathi O (2007) Different doses of sublingual misoprostol versus methylergometrine for the prevention of atonic postpartum haemorrhage. Int J Health Sci (Qassim). 1:229–236
Hofmeyr GJ, Gulmezoglu AM, Novikova N, Linder V, Ferreira S, Piaggio G (2009) Misoprostol to prevent and treat postpartum haemorrhage: a systematic review and meta-analysis of maternal deaths and dose-related effects. Bull World Health Organ 87:666–677
Tang OS, Schweer H, Seyberth HW, Lee SW, Ho PC (2002) Pharmacokinetics of different routes of administration of misoprostol. Hum Reprod 17:332–336
El Tahan MR, Warda OM, Rashad A, Yasseen AM, Ramzy EA, Ahmady MS, Diab DG, Matter MK (2012) Effects of preoperative sublingual misoprostol on uterine tone during isoflurane anesthesia for cesarean section. Rev Bras Anestesiol 62:625–635
Ragab A, Barakat R, Alsammani MA (2016) A randomized clinical trial of preoperative versus postoperative misoprostol in elective cesarean delivery. Int J Gynaecol Obstet 132:82–84
Elsedeek MS (2012) Impact of preoperative rectal misoprostol on blood loss during and after elective cesarean delivery. Int J Gynaecol Obstet 118:149–152
Rane A, Patole S, Singh M, Naidoo D, Buttener P (2003) Intravaginal Prostin gel prior to elective cesarean section at term to induce catecholamine surge in cord blood—a randomised, placebo controlled pilot study. Aust N Z J Obstet Gynecol 43:176
Singh M, Patole S, Rane A, Naidoo D, Buettner P (2004) Maternal intravaginal prostaglandin E2 gel before elective caesarean section at term to induce catecholamine surge in cord blood: randomised, placebo controlled study. Arch Dis Child Fetal Neonatal Ed 89:F131–135
National Collaborating Center for Women’s and Children’s Health (2011) Cesarean section: NICE clinical guideline. RCOG Press, London
Phelan JP, Smith CV, Broussard P, Small M (1987) Amniotic fluid volume assessment with the four-quadrant technique at 36–42 weeks’ gestation. J Reprod Med 32:540–542
Lapaire O, Schneider MC, Stotz M, Surbek DV, Holzgreve W, Hoesli IM (2006) Oral misoprostol vs. intravenous oxytocin in reducing blood loss after emergency cesarean delivery. Int J Gynecol Obstet 95:2–7
Chaudhuri P, Banerjee GB, Mandal A (2010) Rectally administered misoprostol versus intravenous oxytocin infusion during cesarean delivery to reduce intraoperative and postoperative blood loss. Int J Gynecol Obstet 109:25–29
Vimala N, Mittal S, Kumar S (2006) Sublingual misoprostol versus oxytocin infusion to reduce blood loss at cesarean section. Int J Gynecol Obstet 92:106–110
Tang OS, Ho PC (2006) The pharmacokinetics and different regimens of misoprostol in early first-trimester medical abortion. Contraception 74:26–30
Khan RU, El-Refaey H (2003) Pharmacokinetics and adverse-effect profile of rectally administered misoprostol in the third stage of labor. Obstet Gynecol 101:968–974
Owonikoko KM, Arowojolu AO, Okunlola MA (2011) Effect of sublingual misoprostol versus intravenous oxytocin on reducing blood loss at cesarean section in Nigeria: a randomized controlled trial. J Obstet Gynaecol Res 37:715–721
Zhao Y, Li X, Peng Y (1998) [Clinical study on reduction of postpartum bleeding in cesarean section by misoprostol] (Article in Chinese). Zhonghua Fu Chan Ke Za Zhi 33:403–405
Fawole AO, Sotiloye OS, Hunyinbo KI, Umezulike AC, Okunlola MA, Adekanle DA, Osamor J, Adeyanju O, Olowookere OO, Adekunle AO, Singata M, Mangesi L, Hofmeyr GJ (2011) A double-blind, randomized, placebo-controlled trial of misoprostol and routine uterotonics for the prevention of postpartum hemorrhage. Int J Gynaecol Obstet 112:107–111
Bhullar A, Carlan SJ, Hamm J, Lamberty N, White L, Richichi K (2004) Buccal misoprostol to decrease blood loss after vaginal delivery: a randomized trial. Obstet Gynecol 104:1282–1288
Fekih M, Jnifene A, Fathallah K, Ben Regaya L, Memmi A, Bouguizene S, Chaieb A, Bibi M, Khairi H (2009) Intérêt du misoprostol dans la prévention de l’hémorragie du post-partum immédiat en cas de césarienne: essai prospectif randomisé [Benefit of misoprostol for prevention of postpartum hemorrhage in cesarean section: a randomized controlled trial] (Article in French). J Gynecol Obstet Biol Reprod (Paris) 38:588–593
Elati A, Weeks A (2012) Risk of fever after misoprostol for the prevention of postpartum hemorrhage: a meta-analysis. Obstet Gynecol 120:1140–1148
Acknowledgements
This work was supported by Ain-Shams University.
Funding
This study did not receive any funds from any organization.
Author information
Authors and Affiliations
Contributions
MS: protocol/project development, data analysis, and manuscript writing/editing. ME-S: protocol/project development. AA-G: protocol/project development. HE-S: data collection or management. MA-H: data collection or management. HH: data collection or management. AMM: data collection or management. MES: data collection or management. MAE-S: data collection or management. RMM: data collection or management.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Approval was obtained from the ethical committee of the Department of Obstetrics and Gynecology, Ain-Shams University (Approval Number: 3458).
Informed consent
Informed consent was obtained from all individual participants included in the study.
About this article
Cite this article
Sweed, M.S., El-Saied, M.M., Abou-Gamrah, A.E. et al. RETRACTED ARTICLE: Rectal vs. sublingual misoprostol before cesarean section: double-blind, three-arm, randomized clinical trial. Arch Gynecol Obstet 298, 1115–1122 (2018). https://doi.org/10.1007/s00404-018-4894-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-018-4894-2