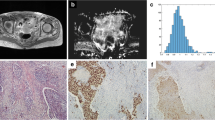
Abstract
Purpose
To retrospectively explore the value of apparent diffusion coefficient (ADC) histogram in assessing local aggressiveness of cervical cancer.
Methods
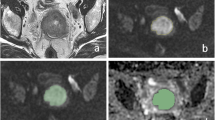
53 patients with cervical cancer, including 7 cases at stage IB1, 17 cases at stage IB2 and 29 cases at stage IIA, were subjected to preoperative MRI including diffusion-weighted imaging with b values of 0 and 800 s/mm2. The average of mean ADC values (ADCmean), minimum ADC values (ADCmin) and the 5th to 85th percentile ADC values every 10 % (ADC5 %, ADC15 %, ADC85 %) were measured. ADC values were compared between subgroups according to pathologic subtype, histological differentiation, depth of cervical infiltration, and lymph node metastases.
Results
ADCmean and ADCmin for adenocarcinoma were 1,170.3 ± 97.8 × 10−6 and 748.7 ± 157.5 × 10−6 mm2 s−1, respectively, significantly higher than that of squamous cell carcinoma (SCC) (1,053.8 ± 134.3 × 10−6 and 615.6 ± 170.2 × 10−6 mm2 s−1, respectively). ADCmean and ADC5 %–ADC85 % of well or moderately tumor were significantly higher than poorly differentiated tumor, but ADCmin was not significantly different among different differentiated cervical cancer. Only ADC5 %–ADC45 % could discriminate well or moderately differentiated SCC from poorly differentiated SCC. ADC5 % for distinguishing well/moderately from poorly differentiated cervical cancer had a largest AUC (0.83). There was no statistical difference in ADC value for different depth of cervical infiltration or lymph node metastases.
Conclusions
ADC values are helpful in assessing pathologic subtype and the differentiation of cervical cancer.



Similar content being viewed by others
References
Orfanoudaki IM, Kappou D, Sifakis S (2011) Recent advances in optical imaging for cervical cancer detection. Arch Gynecol Obstet 284:1197–1208
Tong T, Yajia G, Huaying W, Weijun P (2012) Application of 1.5 T magnetic resonance imaging in endometrial cancer. Arch Gynecol Obstet 285:1113–1118
Biewenga P, van der Velden J, Mol BW, Stalpers LJ, Schilthuis MS, van der Steeg JW, Burger MP, Buist MR (2011) Prognostic model for survival in patients with early stage cervical cancer. Cancer 117:768–776
Stenstedt K, Hellström AC, Fridsten S, Blomqvist L (2011) Impact of MRI in the management and staging of cancer of the uterine cervix. Acta Oncol 50:420–426
Duncan KA, Drinkwater KJ, Frost C, Remedios D, Barter S (2012) Staging cancer of the uterus: a national audit of MRI accuracy. Clin Radiol 67:523–530
Hamstra DA, Rehemtulla A, Ross BD (2007) Diffusion magnetic resonance imaging: a biomarker for treatment response in oncology. J Clin Oncol 25:4104–4109
Punwani S (2011) Diffusion weighted imaging of female pelvic cancers: concepts and clinical applications. Eur J Radiol 78:21–29
Li S, Sun F, Jin ZY et al (2007) Whole-body diffusion-weighted imaging: technical improvement and preliminary results. J Magn Reson Imaging 26:1139–1144
Nakamura K, Joja I, Nagasaka T et al (2012) The mean apparent diffusion coefficient value (ADCmean) on primary cervical cancer is a predictive marker for disease recurrence. Gynecol Oncol 127:478–483
Tozer DJ, Jäger HR, Danchaivijitr N et al (2007) Apparent diffusion coefficient histograms may predict low-grade glioma subtype. NMR Biomed 20:49–57
Costantini M, Belli P, Rinaldi P et al (2010) Diffusion-weighted imaging in breast cancer: relationship between apparent diffusion coefficient and tumour aggressiveness. Clin Radiol 65:1005–1012
Itou Y, Nakanishi K, Narumi Y, Nishizawa Y, Tsukuma H (2011) Clinical utility of apparent diffusion coefficient (ADC) values in patients with prostate cancer: can ADC values contribute to assess the aggressiveness of prostate cancer? J Magn Reson Imaging 33:167–172
Barajas RF Jr, Rubenstein JL, Chang JS, Hwang J, Cha S (2010) Diffusion-weighted MR imaging derived apparent diffusion coefficient is predictive of clinical outcome in primary central nervous system lymphoma. AJNR Am J Neuroradiol 31:60–66
Liu Y, Bai R, Sun H, Liu H, Wang D (2009) Diffusion-weighted magnetic resonance imaging of uterine cervical cancer. J Comput Assist Tomogr 33:858–862
Payne GS, Schmidt M, Morgan VA et al (2010) Evaluation of magnetic resonance diffusion and spectroscopy measurements as predictive biomarkers in stage 1 cervical cancer. Gynecol Oncol 116:246–252
Kang Y, Choi SH, Kim YJ et al (2011) Gliomas: histogram analysis of apparent diffusion coefficient maps with standard- or high-b-value diffusion-weighted MR imaging—correlation with tumor grade. Radiology 261:882–890
Kyriazi S, Collins DJ, Messiou C et al (2011) Metastatic ovarian and primary peritoneal cancer: assessing chemotherapy response with diffusion-weighted MR imaging—value of histogram analysis of apparent diffusion coefficients. Radiology 26:182–192
McVeigh PZ, Syed AM, Milosevic M, Fyles A, Haider MA (2008) Diffusion-weighted MRI in cervical cancer. Eur Radiol 18:1058–1064
Abu-Rustum NR, Alektiar K, Iasonos A et al (2006) The incidence of symptomatic lower-extremity lymphedema following treatment of uterine corpus malignancies: a 12-year experience at Memorial Sloan-Kettering Cancer Center. Gynecol Oncol 103:714–718
Alexander-Sefre F, Chee N, Spencer C et al (2006) Surgical morbidity associated with radical trachelectomy and radical hysterectomy. Gynecol Oncol 101:450–454
Pieterse QD, Maas CP, ter Kuile MM et al (2006) An observational longitudinal study to evaluate miction, defecation, and sexual function after radical hysterectomy with pelvic lymphadenectomy for earlystage cervical cancer. Int J Gynecol Cancer 16:1119–1129
Meirovitz M, Sade S, Dreiher J et al (2013) Is radical hysterectomy necessary in early cervical cancer? Gynecol Obstet Invest 76:158–162
Pluta M, Rob L, Charvat M et al (2009) Less radical surgery than radical hysterectomy in early stage cervical cancer: a pilot study. Gynecol Oncol 113:181–184
Kim MK, Kim JW, Kim MA et al (2010) Feasibility of less radical surgery for superficially invasive carcinoma of the cervix. Gynecol Oncol 119:187–191
Kodama J, Kusumoto T, Nakamura K et al (2011) Factors associated with parametrial involvement in stage IB1 cervical cancer and identification of patients suitable for less radical surgery. Gynecol Oncol 122:491–494
Motoshima S, Irie H, Nakazono T, Kamura T, Kudo S (2011) Diffusion-weighted MR imaging in gynecologic cancers. J Gynecol Oncol 22:275–287
Zou QG, Xu HB, Liu F, Guo W, Kong XC, Wu Y (2011) In the assessment of supratentorial glioma grade: the combined role of multivoxel proton MR spectroscopy and diffusion tensor imaging. Clin Radiol 66:953–960
Curvo-Semedo L, Lambregts DM, Maas M, Beets GL, Caseiro-Alves F, Beets-Tan RG (2012) Diffusion-weighted MRI in rectal cancer: apparent diffusion coefficient as a potential noninvasive marker of tumor aggressiveness. J Magn Reson Imaging 35:1365–1371
Nishie A, Tajima T, Asayama Y et al (2011) Diagnostic performance of apparent diffusion coefficient for predicting histological grade of hepatocellular carcinoma. Eur J Radiol 80:e29–e33
Razek AA, Fathy A, Gawad TA (2011) Correlation of apparent diffusion coefficient value with prognostic parameters of lung cancer. J Comput Assist Tomogr 35:248–252
Matoba M, Tonami H, Kondou T et al (2007) Lung carcinoma: diffusion-weighted mr imaging—preliminary evaluation with apparent diffusion coefficient. Radiology 243:570–577
Dzik-Jurasz A, Domenig C, George M et al (2002) Diffusion MRI for prediction of response of rectal cancer to chemoradiation. Lancet 27:307–308
Sugahara T, Korogi Y, Kochi M et al (1999) Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging 9:53–60
Verma S, Rajesh A, Morales H et al (2011) Assessment of aggressiveness of prostate cancer: correlation of apparent diffusion coefficient with histologic grade after radical prostatectomy. AJR Am J Roentgenol 196:374–381
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
H. Xue and C. Ren contributed equally to this work.
Rights and permissions
About this article
Cite this article
Xue, H., Ren, C., Yang, J. et al. Histogram analysis of apparent diffusion coefficient for the assessment of local aggressiveness of cervical cancer. Arch Gynecol Obstet 290, 341–348 (2014). https://doi.org/10.1007/s00404-014-3221-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-014-3221-9