Abstract
Mycosis fungoides (MF) is the most common primary cutaneous T-cell lymphoma (CTCL) with its etiology not yet fully understood. Interleukin (IL)-35 is an inhibitory cytokine that belongs to the IL-12 family. Elevated IL-35 in the plasma and the tumor microenvironment increases tumorigenesis and indicates poor prognosis in different types of malignancies. The objective of this study is to estimate the expression levels of IL-35 in tissue and serum of MF patients versus healthy controls. This case-control study included 35 patients with patch, plaque, and tumor MF as well as 30 healthy controls. Patients were fully assessed, and serum samples and lesional skin biopsies were taken prior to starting treatment. The IL-35 levels were measured in both serum and tissue biopsies by ELISA technique. Both tissue and serum IL-35 levels were significantly higher in MF patients than in controls (P < 0.001) and tissue IL-35 was significantly higher than serum IL-35 in MF patients (P < 0.001). Tissue IL-35 was significantly higher in female patients and patients with recurrent MF compared to male patients and those without recurrent disease (P < 0.001). Since both tissue and serum IL-35 levels are increased in MF, IL-35 is suggested to have a possible role in MF pathogenesis. IL-35 can be a useful diagnostic marker for MF. Tissue IL-35 can also be an indicator of disease recurrence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The most frequent form of primary cutaneous lymphoma is mycosis fungoides (MF). lt is postulated to derive from mature skin-resident effector memory T cells [1]. The majority of patients with MF are patch and plaque phases, and a minority advance to the tumor stage. MF often has a protracted and indolent course [2].
The etiopathogenesis of CTCL remains largely unknown. Genomic analysis of CTCL cell lines has shown genetic, epigenetic, and chromosomal aberrations that can affect lymphomagenesis and consequently, disease progression. The uncontrolled clonal proliferation of atypical lymphocytes results from chronic antigenic stimulation, together with the underlying cytogenetic abnormalities [3]. Neoplastic T-cell clones in CTCL secrete Th2-associated cytokines that lead to progressive immune dysregulation and tumor growth [4].
Interleukin-35 (IL-35) is an inhibitory cytokine belonging to the IL-12 family. It can suppress T cell proliferation and induce IL-35-producing induced-regulatory T cells (iTr35) which inhibits inflammatory responses [5].
IL-35 was thought to be produced by activated Tregs but more recent studies revealed the expression of IL-35 by other immunoregulatory cells as regulatory B cells (Bregs), tolerogenic DCs (tolDCs), prometastatic tumor-associated macrophages, and CD8 + T regulatory cells [5].
The effect of IL-35 on the neoplastic cells and the relationship between its expression rate and different stages of cancer is currently unknown [6]. High levels of IL‐35 in the plasma tumor microenvironment increase tumorigenesis and denote a poor prognosis in many neoplasms [7].
IL-35 increases tumorigenesis by inhibiting the antitumor activity of lymphocytes which leads to the accumulation of CD11b + Gr1 + myeloid cells (MDSC), also tumor-produced IL‐35 has an indirect role in MDSC chemotaxis. The elevated MDSC produces vascular endothelial growth factor (VEGF) together with other factors that promote tumor angiogenesis and tumor cell expansion [6]. In addition, IL-35 in tumor-infiltrating Treg cells can promote the expression of programmed cell death protein-1 (PD-1) on the surface of the tumor T cells, leading to their exhaustion [8].
Subjects and methods
This study was designed as a case-control study. It was conducted at the Dermatology outpatient clinic, Dermatology department, Faculty of Medicine, Cairo University Hospital (Kasr Al Ainy) from December 2022 to June 2023. The Dermatology Research Ethical Committee had approved this study’s protocol. Confidentiality in handling the data was guaranteed. A written informed consent was signed by each patient participating in the study. Clinical trial ID: NCT05855460.
Patients and controls
Thirty-five MF patients (≥ 18 years old) including both sexes, were recruited, as well as 30 age- and sex-matched healthy controls.
The diagnosis of MF was confirmed histopathologically by two skin biopsies and patients were properly clinically assessed and investigated by chest X-ray, abdominal, and lymph node ultrasonography for staging their MF by the TNM staging. All patients with classic MF including patch, plaque, and tumor stages were included. Patients with any other skin disease, patients receiving topical treatment, systemic treatment, or phototherapy for MF during the past 3 months and patients with a history of a solid or hematological malignancy were excluded from this study.
Methods
For assessment of tissue levels of IL35, a 4 mm punch skin biopsy was taken from every patient’s lesional skin and healthy control and kept incubated in phosphate-buffered saline (PBS) at -80 degrees till assayed. For assessment of serum levels of IL35, 5 ml venous blood was taken from every patient and healthy control and centrifuged at 8000 RPM for 20 min, and then serum was separated and kept frozen at -80 degrees till assayed. The serum and tissue levels of Human Interleukin-35 were assayed by a commercially available ELISA kit supplied by SUNLONG BIOTECH Co., Ltd., China. Catalog No. SL1009Hu. Details of laboratory methods and statistical analysis are illustrated in the supplementary file.
Results
This study included 35 MF patients and 30 healthy controls. Both groups were age- and sex-matched (P > 0.05). MF patients included 15 male (42.9%) and 20 female (57.1%) patients, and their ages ranged between 22 and 73 years with a mean of 48.69 ± 14.79 years. Meanwhile, healthy controls included 16 females (53.3%) and 14 males (46.7%) and their ages ranged between 20 and 68 years with a mean of 42.47 ± 14.81 years. The clinical data of patients are shown in Table 1, and the laboratory investigations of patients are illustrated in Suppl. Table 1.
The levels of both tissue and serum levels of IL-35 were significantly higher in MF patients than in healthy controls (P < 0.001) (Table 2) (Fig. 1).
A receiver operator characteristic (ROC) curve was done to detect the cutoff value, sensitivity, and specificity of tissue and serum IL-35. Both were found as a significant diagnostic marker of MF (P < 0.001) with tissue IL-35 area under the curve (AUC) was 1.000 (cutoff value 2.5318 ng/ml, 95% confidence interval 1.000–1.000 with sensitivity 100% and specificity 100%) and serum IL-35 AUC was 1.000 (cutoff value 1.0036 ng/ml, 95% confidence interval 1.000–1.000 with sensitivity 100% and specificity 100%). The positive predictive value (PPV) was 100%, The negative predictive value (NPV) was 100% and the accuracy was 100%. The results demonstrate that both IL-35 tissue, and serum levels have excellent diagnostic accuracy, as indicated by the AUC of 1.00. The high sensitivity, specificity, PPV, NPV, and accuracy values further support the reliability of IL- 35 levels as a diagnostic marker (Suppl. Table 2).
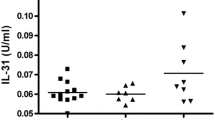
Regarding IL-35 tissue level in the patients’ group, a statistically significant difference was found between males and females (P < 0.001), with higher levels in females (Fig. 2). Also, the difference in the level between patients with and without recurrent disease was statistically significant (P < 0.001) with a higher level in patients with recurrent disease (Fig. 3). However, no statistically significant differences were found in IL-35 tissue level among different stages, type of lesions, or different skin types (P > 0.05) (Suppl. Table 3).
Regarding IL-35 serum levels in the patients’ group, no statistically significant differences were found in IL-35 serum level and sex, skin types, among different stages, types of lesions, or recurrent disease (P˃0.05) (Suppl. Table 4).
No correlations were found between either IL-35 tissue or serum levels and age (of patients or controls), duration of disease, or BSA % (P˃0.05). Also, no statistically significant difference was found in either IL-35 tissue or serum levels between males and females in the control group (P > 0.05) (Suppl. Tables 5,6).
Regarding patients’ laboratory investigations, IL-35 tissue level showed only a statistically significant negative correlation with monocyte count (P = 0.014, r=-0.412). Also, there was a statistically significant positive correlation between IL- 35 serum level and platelet count (P = 0.008, r = 0.442). No correlations were found between either tissue or serum IL-35 and any other laboratory investigation (P˃0.05) (Suppl. Tables 5,6).
A statistically significant positive correlation was found between tissue and serum IL-35 levels in controls (P = 0.006, r = 0.491) (Fig. 4). This positive correlation was not significant among the MF group.
Discussion
The mechanisms involved in the progression of MF remain unclear. Different factors could be involved such as aberrant molecular expression, overexpression of microRNA, changes in release of cytokines, genetic mutations, and different compositions of cells in the tumor microenvironment [9].
Tumor microenvironment changes are due to the production of different cytokines from the tumor cells. This eventually leads to a shift in the immune response from an anti-tumor (Th1) to a tumorigenic (Th2) one [10]. This shift will increase the immunosuppressive cytokines (IL-2, IL-4, IL-7, IL-13, and IL-15) production by neoplastic elements which lead to tumor growth and spread [11].
Interleukin-35 (IL-35) is an IL-12 family cytokines member, but unlike other IL-12 family members, it has immuno-suppressive activity [12].
The immune-suppressive cytokines lead to alteration in the epigenome and the transcriptional network in naïve immune cells [13]. These cytokines act on cytotoxic CD8 + T cells or natural killer (NK) cells and inhibit their effector functions and proliferation into the tumor microenvironment [14]. The immunosuppressive cytokines, TGF-β, IL-4, IL-10, and IL-35, are the cytokines that are dominant in the tumor environment in different tumors. Targeting these cytokines can limit the survival of tumor cells and control tumor growth and metastasis [15].
The role of IL-35 in MF has not been studied before; accordingly, this study was designed to assess the IL-35 level in lesional skin and serum of 35 MF patients versus 30 healthy controls and statistically significant differences between the studied groups were detected regarding the levels of tissue and serum IL-35. These levels were found to be significantly higher in MF patients than in the healthy controls. These findings were augmented by unadjusted and adjusted regression analysis which proved that both serum and tissue IL-35 are significant diagnostic markers for MF.
Our results are in concordance with the findings of previous studies that had detected elevated levels of IL-35 in different tumors such as diffuse large B-cell lymphoma [16], acute myeloid leukemia [17], acute lymphoblastic leukemia [18], colorectal carcinoma [19], pancreatic adenocarcinoma [20], gastric carcinoma [21, 22], prostate carcinoma [23], breast carcinoma [24], non-small cell lung cancer [25], and hepatocellular carcinoma [26].
Interleukin-35 also inhibits immune responses by promoting regulatory T cells (Tregs) and regulatory B cells (Bregs) expansion and at the same time suppressing macrophages, effector T cells, Th1 cells, and Th17 cells [27]. Also, IL-35 has been found to affect the secretion of other cytokines such as IL-6, granulocyte colony-stimulating factor (G-CSF), and IFN-γ eventually leading to a protumor effect [27].
Although higher levels of IL-35 were linked to disease progression and metastasis in other studies, our study didn’t find statistically significant differences between different stages of MF. This may be due to the low number of patients in advanced stages included in the study. So, further studies are needed with a larger number of patients in advanced stages to assess its prognostic value, especially patients with blood involvement.
Moreover, a study revealed that recombinant IL-35 stimulates PD-1 in peripheral CD8 + T cells as well as those infiltrating the tumour in patients with hepatocellular carcinoma (HCC). This shows that IL-35 and its antibody could be potential therapeutic agents in different neoplasms and immune-mediated diseases [28].
In the current study, tissue levels of IL-35 were significantly higher in females. This is mostly related to high estrogen levels. Polanczyk et al. [29] reported that estrogen has immunomodulatory and immunosuppressive functions by reducing activation of effector T cells, potentiation of Treg cells, inhibiting the induction of the inflammatory cytokines as IL-12 and interferon-gamma but enhancing the secretion of the immunosuppressive cytokines as IL-10 and enhancing the expression of the PD-1 costimulatory pathway.
Moreover, the level of tissue IL-35 was significantly higher in patients with recurrent MF. The higher level of IL-35 in relapsing disease may be related to the clonal expansion of malignant T cells and the dysregulated immune response in relapsing cancers. In MF, the malignant T cells evade immune surveillance through different mechanisms such as downregulation of major histocompatibility complex molecules, impaired antigen presentation, and the production of immunosuppressive cytokines. These immune evasion strategies allow the malignant T cells to escape recognition and elimination by the immune system, contributing to disease relapse [30]. Therefore, tissue IL-35 may be of value as a potential marker to pick up very early recurring cases.
Regarding patients’ laboratory investigations, IL-35 tissue level showed a statistically significant negative correlation with monocyte count. In CTCL, macrophage-related chemokines and angiogenic factors produced by tumor-associated macrophages (TAMs), developing from tissue-infiltrating monocytes, have crucial roles in tumor formation in the lesional skin of MF. The TAMs maintain an immunosuppressive tumor microenvironment by recruiting Tregs, circulating myeloid cells, and myeloid-derived suppressor cells [31]. In the same context, both subunits of IL-35 (EBI3 and IL-12a) are highly expressed in metastatic TAMs [32].
Also, there was a statistically significant positive correlation between IL- 35 serum level and platelet count. This could be attributed to the active role of platelets (rich in angiogenic factors in their granules) in the tumor microenvironment (TME), being involved in tumor angiogenesis as they are rich in angiogenic factors in their granules [33]. Similarly, IL-35 promotes tumor angiogenesis and tumor cell expansion through inducing the formation of VEGF [6].
No correlations were found between either tissue or serum IL-35 and any other laboratory investigation (P˃0.05) although some studies reported significant correlations suggesting MF association with metabolic syndrome and cardiovascular risk [34, 35].
Also, a statistically significant difference was detected between the level of tissue and serum IL-35 in patients, with higher levels in tissue. This may point out that tissue IL-35 level is more accurate as a diagnostic marker in MF than the serum level.
Conclusion
In conclusion, being significantly higher in MF patients compared to healthy controls, IL-35 is suggested to play a possible role in MF pathogenesis. IL-35 can be a useful diagnostic marker in MF. Our study found that tissue IL-35 was significantly higher in patients with recurrent MF compared to de novo disease. So, tissue IL-35 can also be an early indicator for disease recurrence in suspicious lesions.
In line with the promising use of anti-IL-35 antibodies in different cancers, anti-IL-35 antibodies may have a promising role as a potential therapeutic agent for MF.
Data availability
The data supporting findings of this study are available within the article. Raw data from which the findings of this study were obtained, are available from the corresponding author, upon request.
References
Campbell JJ, Clark RA, Watanabe R, Kupper TS (2010) Sézary syndrome and mycosis fungoides arise from distinct T-cell subsets: a biologic rationale for their distinct clinical behaviors. Blood 116:767–771. https://doi.org/10.1182/blood-2009-11-251926
Kamijo H, Miyagaki T (2021) Mycosis Fungoides and Sézary Syndrome: updates and review of current therapy. Curr Treat Options Oncol 22:10. https://doi.org/10.1007/s11864-020-00809-w
Martinez XU, Di Raimondo C, Abdulla FR, Zain J, Rosen ST, Querfeld C (2019) Leukaemic variants of cutaneous T-cell lymphoma: erythrodermic mycosis fungoides and Sézary syndrome. Best Pract Res Clin Haematol 32:239–252. https://doi.org/10.1016/j.beha.2019.06.004
Patil K, Kuttikrishnan S, Khan AQ, Ahmad F, Alam M, Buddenkotte J, Ahmad A, Steinhoff M, Uddin S (2022) Molecular pathogenesis of cutaneous T cell lymphoma: role of chemokines, cytokines, and dysregulated signaling pathways. Semin Cancer Biol 86:382–399. https://doi.org/10.1016/j.semcancer.2021.12.003
Ye C, Yano H, Workman CJ, Vignali DAA (2021) Interleukin-35: structure, function and its impact on Immune-Related diseases. J Interf Cytokine Res 41:391–406. https://doi.org/10.1089/jir.2021.0147
Yazdani Z, Rafiei A, Golpour M, Zafari P, Moonesi M, Ghaffari S (2020) IL-35, a double‐edged sword in cancer. J Cell Biochem 121:2064–2076. https://doi.org/10.1002/jcb.29441
Nishino R, Takano A, Oshita H, Ishikawa N, Akiyama H, Ito H, Nakayama H, Miyagi Y, Tsuchiya E, Kohno N, Nakamura Y, Daigo Y (2011) Identification of Epstein-Barr Virus–Induced Gene 3 as a Novel serum and tissue biomarker and a therapeutic target for Lung Cancer. Clin Cancer Res 17:6272–6286. https://doi.org/10.1158/1078-0432.CCR-11-0060
Turnis ME, Sawant DV, Szymczak-Workman AL, Andrews LP, Delgoffe GM, Yano H, Beres AJ, Vogel P, Workman CJ, Vignali DAA (2016) Interleukin-35 limits Anti-tumor Immunity. Immunity 44:316–329. https://doi.org/10.1016/j.immuni.2016.01.013
Pileri A, Guglielmo A, Grandi V, Violetti SA, Fanoni D, Fava P, Agostinelli C, Berti E, Quaglino P, Pimpinelli N (2021) The Microenvironment’s role in Mycosis Fungoides and Sézary Syndrome: from progression to therapeutic implications. Cells 10:2780. https://doi.org/10.3390/cells10102780
Krejsgaard T, Lindahl LM, Mongan NP, Wasik MA, Litvinov IV, Iversen L, Langhoff E, Woetmann A, Odum N (2017) Malignant inflammation in cutaneous T-cell lymphoma—a hostile takeover. Semin Immunopathol 39:269–282. https://doi.org/10.1007/s00281-016-0594-9
Karpova MB, Fujii K, Jenni D, Dummer R, Urosevic-Maiwald M (2011) Evaluation of lymphangiogenic markers in Sézary syndrome. Leuk Lymphoma 52:491–501. https://doi.org/10.3109/10428194.2010.517877
Teymouri M, Pirro M, Fallarino F, Gargaro M, Sahebkar A (2018) IL-35, a hallmark of immune‐regulation in cancer progression, chronic infections and inflammatory diseases. Int J Cancer 143:2105–2115. https://doi.org/10.1002/ijc.31382
Zamarron BF, Chen W (2011) Dual Roles of Immune Cells and their factors in Cancer Development and Progression. Int J Biol Sci 7:651–658. https://doi.org/10.7150/ijbs.7.651
Uzhachenko RV, Shanker A (2019) CD8 + T lymphocyte and NK Cell Network: Circuitry in the cytotoxic domain of immunity. Front Immunol 10. https://doi.org/10.3389/fimmu.2019.01906
Mirlekar B (2022) Tumor promoting roles of IL-10, TGF-β, IL-4, and IL-35: its implications in cancer immunotherapy. SAGE Open Med 10:205031212110690. https://doi.org/10.1177/20503121211069012
Larousserie F, Kebe D, Huynh T, Audebourg A, Tamburini J, Terris B, Devergne O (2019) Evidence for IL-35 expression in diffuse large B-Cell lymphoma and impact on the patient’s prognosis. Front Oncol 9. https://doi.org/10.3389/fonc.2019.00563
Wang J, Tao Q, Wang H, Wang Z, Wu F, Pan Y, Tao L, Xiong S, Wang Y, Zhai Z (2015) Elevated IL-35 in bone marrow of the patients with acute myeloid leukemia. Hum Immunol 76:681–686. https://doi.org/10.1016/j.humimm.2015.09.020
Solati H, Zareinejad M, Ghavami A, Ghasemi Z, Amirghofran Z (2020) IL-35 and IL-18 serum levels in children with Acute Lymphoblastic Leukemia: the relationship with prognostic factors. J Pediatr Hematol Oncol 42:281–286. https://doi.org/10.1097/MPH.0000000000001667
Zeng J-C, Zhang Z, Li T-Y, Liang Y-F, Wang H-M, Bao J-J, Zhang J-A, Wang W-D, Xiang W-Y, Kong B, Wang Z-Y, Wu B-H, Chen X-D, He L, Zhang S, Wang C-Y, Xu J-F (2013) Assessing the role of IL-35 in colorectal cancer progression and prognosis. Int J Clin Exp Pathol 6:1806–1816
Jin P, Ren H, Sun W, Xin W, Zhang H, Hao J (2014) Circulating IL-35 in pancreatic ductal adenocarcinoma patients. Hum Immunol 75:29–33. https://doi.org/10.1016/j.humimm.2013.09.018
Fan Y-G, Zhai J-M, Wang W, Feng B, Yao G-L, An Y-H, Zeng C (2015) IL-35 over-expression is Associated with Genesis of Gastric Cancer. Asian Pac J Cancer Prev 16:2845–2849. https://doi.org/10.7314/APJCP.2015.16.7.2845
Gu J, Wang X, Wang L, Zhou L, Tang M, Li P, Wu X, Chen M, Zhang Y (2021) Serum level of interleukin-35 as a potential prognostic factor for gastric cancer. Asia Pac J Clin Oncol 17:52–59. https://doi.org/10.1111/ajco.13403
Zhu J, Yang X, Wang Y, Zhang H, Guo Z (2019) Interleukin–35 is associated with the tumorigenesis and progression of prostate cancer. Oncol Lett. https://doi.org/10.3892/ol.2019.10208
Chen G, Liang Y, Guan X, Chen H, Liu Q, Lin B, Chen C, Huang M, Chen J, Wu W, Liang Y, Zhou K, Zeng J (2016) Circulating low IL-23: IL-35 cytokine ratio promotes progression associated with poor prognosisin breast cancer. Am J Transl Res 8:2255–2264
Zhang T, Nie J, Liu X, Han Z, Ding N, Gai K, Liu Y, Chen L, Guo C (2021) Correlation analysis among the level of IL-35, Microvessel Density, Lymphatic Vessel density, and prognosis in non‐small cell Lung Cancer. Clin Transl Sci 14:389–394. https://doi.org/10.1111/cts.12891
Liu X, Ren H, Guo H, Wang W, Zhao N (2021) Interleukin-35 has a tumor-promoting role in hepatocellular carcinoma. Clin Exp Immunol 203:219–229. https://doi.org/10.1111/cei.13535
Liu K, Huang A, Nie J, Tan J, Xing S, Qu Y, Jiang K (2021) IL-35 regulates the function of Immune cells in Tumor Microenvironment. Front Immunol 12. https://doi.org/10.3389/fimmu.2021.683332
Yang L, Shao X, Jia S, Zhang Q, Jin Z (2019) Interleukin-35 dampens CD8 + T cells activity in patients with non-viral Hepatitis-Related Hepatocellular Carcinoma. Front Immunol 10. https://doi.org/10.3389/fimmu.2019.01032
Polanczyk MJ, Hopke C, Vandenbark AA, Offner H (2006) Estrogen-mediated immunomodulation involves reduced activation of effector T cells, potentiation of treg cells, and enhanced expression of the PD-1 costimulatory pathway. J Neurosci Res 84:370–378. https://doi.org/10.1002/jnr.20881
Upadhyay R, Hammerich L, Peng P, Brown B, Merad M, Brody J (2015) Lymphoma: Immune Evasion Strategies. Cancers (Basel) 7:736–762. https://doi.org/10.3390/cancers7020736
Furudate S, Fujimura T, Kakizaki A, Hidaka T, Asano M, Aiba S (2016) Tumor-associated M2 macrophages in mycosis fungoides acquire immunomodulatory function by interferon alpha and interferon gamma. J Dermatol Sci 83:182–189. https://doi.org/10.1016/j.jdermsci.2016.05.004
Heim L, Kachler K, Siegmund R, Trufa DI, Mittler S, Geppert C-I, Friedrich J, Rieker RJ, Sirbu H, Finotto S (2019) Increased expression of the immunosuppressive interleukin-35 in patients with non-small cell lung cancer. Br J Cancer 120:903–912. https://doi.org/10.1038/s41416-019-0444-3
Filippelli A, Del Gaudio C, Simonis V, Ciccone V, Spini A, Donnini S (2022) Scoping review on platelets and Tumor angiogenesis: do we need more evidence or Better Analysis? Int J Mol Sci 23:13401. https://doi.org/10.3390/ijms232113401
Youssef R, Hay R, Aboulghate A, Ibrahim N, Hegazy A, Sayed K (2021) Mycosis fungoides and metabolic syndrome: a hospital-based case-control study. J Egypt Women’s Dermatol Soc 18:174. https://doi.org/10.4103/jewd.jewd_10_21
Johnson CM, Talluru SM, Bubic B, Colbert M, Kumar P, Tsai H, Varadhan R, Rozati S (2023) Association of Cardiovascular Disease in patients with Mycosis Fungoides and Sézary Syndrome compared to a Matched Control Cohort. JID Innov 3:100219. https://doi.org/10.1016/j.xjidi.2023.100219
Funding
None.
Conflict of interestNone declared.
Ethics approvalThe Research Ethics Committee of the Faculty of Medicine, Cairo University approved this study.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors contributed to the fulfillment of this study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elmasry, M.F., Obaid, Y.A., El-Samanoudy, S.I. et al. Estimation of the tissue and serum levels of IL-35 in Mycosis fungoides: a case-control study. Arch Dermatol Res 316, 349 (2024). https://doi.org/10.1007/s00403-024-03115-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00403-024-03115-9