Abstract
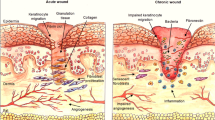
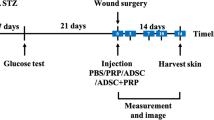
Diabetes mellitus, as an important metabolic disorder, affects the health of millions of people worldwide. A diabetic wound is one of the complications of diabetes. The stem cell secretome can particularly affect the wound healing process in diabetic wounds. The present study aimed to investigate the effects of Adipose-derived stem cells (ASCs) secretome on the skin wound healing process, angiogenesis, and inflammation in diabetic rats. For this purpose, ASCs were extracted from Adipose tissue and confirmed by flow cytometry and cell differentiation. Secretome was prepared. 27 rats were divided into three groups, non-diabetic, diabetic (treated with phosphate-buffered saline), and diabetics treated with secretome. The levels of vascular endothelial growth factor (VEGF) and transforming growth factor-beta (TGF-β) were examined by the enzyme-linked immunosorbent assay (ELISA) was performed in the skin tissues of all groups. Hematoxylin and eosin (H&E) staining was performed. The level of VEGF was higher in the diabetic group treated with secretome as compared to the other two groups, while the level of TGF-β was lower in this group, compared to the diabetic group. Based on the results of H&E staining, the epidermal thickness and angiogenesis were higher in the diabetic group treated with secretome, whereas edema, number of inflammatory cells, and epidermal damage were lower in this group, compared to the diabetic group. Subcutaneous injection of secretome can lead to diabetic wound healing by increasing growth factors associated with angiogenesis such as VEGF, increasing angiogenesis, regulating TGF-β levels, reducing inflammatory cells.






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, [S.H.H], upon reasonable request and with the permission of the publisher.
References
Bai Q, Han K, Dong K et al (2020) Potential applications of nanomaterials and technology for diabetic wound healing. Int J Nanomed 15:9717–9743
An R, Zhang Y, Qiao Y et al (2020) Adipose stem cells isolated from diabetic mice improve cutaneous wound healing in streptozotocin-induced diabetic mice. Stem Cell Res Ther. https://doi.org/10.1186/s13287-020-01621-x
Hamada M, Iwata T, Kato Y et al (2017) Xenogeneic transplantation of human adipose-derived stem cell sheets accelerate angiogenesis and the healing of skin wounds in a Zucker Diabetic Fatty rat model of obese diabetes. Regen Ther 6:65–73. https://doi.org/10.1016/j.reth.2017.02.002
O’Loughlin A, Kulkarni M, Creane M et al (2013) Topical administration of allogeneic mesenchymal stromal cells seeded in a collagen scaffold augments wound healing and increases angiogenesis in the diabetic rabbit ulcer. Diabetes 62:2588–2594. https://doi.org/10.2337/db12-1822
Chen S, Shi J, Zhang M et al (2015) Mesenchymal stem cell-laden anti-inflammatory hydrogel enhances diabetic wound healing. Sci Rep 5:18104. https://doi.org/10.1038/srep18104
Xu J, Zgheib C, Hodges MM et al (2017) Mesenchymal stem cells correct impaired diabetic wound healing by decreasing ECM proteolysis. Physiol Genom 49:541–548. https://doi.org/10.1152/physiolgenomics.00090.2016
Kanji S, Das H (2017) Advances of stem cell therapeutics in cutaneous wound healing and regeneration. Mediators Inflamm. https://doi.org/10.1155/2017/5217967
Trzyna A, Banaś-Ząbczyk A (2021) Adipose-derived stem cells secretome and its potential application in “stem cell-free therapy.” Biomolecules 11:878
Guo X, Schaudinn C, Blume-Peytavi U et al (2022) Effects of adipose-derived stem cells and their conditioned medium in a human ex vivo wound model. Cells 11:1198. https://doi.org/10.3390/cells11071198
An Y-H, Kim DH, Lee EJ et al (2021) High-efficient production of adipose-derived stem cell (ADSC) secretome through maturation process and its non-scarring wound healing applications. Front Bioeng Biotechnol. https://doi.org/10.3389/fbioe.2021.681501
Bari E, Perteghella S, Di Silvestre D et al (2018) Pilot production of mesenchymal stem/stromal freeze-dried secretome for cell-free regenerative nanomedicine: a validated GMP-compliant process. Cells 7:190. https://doi.org/10.3390/cells7110190
Foo JB, Looi QH, Chong PP et al (2021) Comparing the therapeutic potential of stem cells and their secretory products in regenerative medicine. Stem Cells Int 2021:1–30. https://doi.org/10.1155/2021/2616807
Sumarwoto T, Suroto H, Mahyudin F et al (2021) Role of adipose mesenchymal stem cells and secretome in peripheral nerve regeneration. Ann Med Surg. https://doi.org/10.1016/j.amsu.2021.102482
Zhou X, Ning K, Ling B et al (2019) Multiple injections of autologous adipose-derived stem cells accelerate the burn wound healing process and promote blood vessel regeneration in a rat model. Stem Cells Dev 28:1463–1472. https://doi.org/10.1089/scd.2019.0113
Kato Y, Iwata T, Washio K et al (2017) Creation and transplantation of an adipose-derived stem cell (ASC) sheet in a diabetic wound-healing model. J Vis Exp. https://doi.org/10.3791/54539
Azam M, Ghufran H, Butt H et al (2021) Curcumin preconditioning enhances the efficacy of adipose-derived mesenchymal stem cells to accelerate healing of burn wounds. Burns Trauma. https://doi.org/10.1093/burnst/tkab021
Liu J, Qiu X, Lv Y et al (2020) Apoptotic bodies derived from mesenchymal stem cells promote cutaneous wound healing via regulating the functions of macrophages. Stem Cell Res Ther 11:507. https://doi.org/10.1186/s13287-020-02014-w
Enderami SE, Soleimani M, Mortazavi Y et al (2018) Generation of insulin-producing cells from human adipose-derived mesenchymal stem cells on PVA scaffold by optimized differentiation protocol. J Cell Physiol 233:4327–4337. https://doi.org/10.1002/jcp.26266
Park S-R, Kim J-W, Jun H-S et al (2018) Stem cell secretome and its effect on cellular mechanisms relevant to wound healing. Mol Ther 26:606–617. https://doi.org/10.1016/j.ymthe.2017.09.023
Zhang S, Chen L, Zhang G, Zhang B (2020) Umbilical cord-matrix stem cells induce the functional restoration of vascular endothelial cells and enhance skin wound healing in diabetic mice via the polarized macrophages. Stem Cell Res Ther 11:39. https://doi.org/10.1186/s13287-020-1561-x
Bai H, Kyu-Cheol N, Wang Z et al (2020) Regulation of inflammatory microenvironment using a self-healing hydrogel loaded with BM-MSCs for advanced wound healing in rat diabetic foot ulcers. J Tissue Eng 11:204173142094724. https://doi.org/10.1177/2041731420947242
Kiritsi D, Nyström A (2018) The role of TGFβ in wound healing pathologies. Mech Ageing Dev 172:51–58. https://doi.org/10.1016/j.mad.2017.11.004
Wang J, Wu H, Peng Y et al (2021) Hypoxia adipose stem cell-derived exosomes promote high-quality healing of diabetic wound involves activation of PI3K/Akt pathways. J Nanobiotechnol. https://doi.org/10.1186/s12951-021-00942-0
Shin S, Lee J, Kwon Y et al (2021) Comparative proteomic analysis of the mesenchymal stem cells secretome from adipose, bone marrow, placenta and Wharton’s Jelly. Int J Mol Sci. https://doi.org/10.3390/ijms22020845
Hu L, Hu J, Zhao J et al (2013) Side-by-side comparison of the biological characteristics of human umbilical cord and adipose tissue-derived mesenchymal stem cells. Biomed Res Int. https://doi.org/10.1155/2013/438243
Zhang J, Liu Y, Chen Y et al (2020) Adipose-derived stem cells: current applications and future directions in the regeneration of multiple tissues. Stem Cells Int 2020:8810813. https://doi.org/10.1155/2020/8810813
Moriyama M, Sahara S, Zaiki K et al (2019) Adipose-derived stromal/stem cells improve epidermal homeostasis. Sci Rep 9:18371. https://doi.org/10.1038/s41598-019-54797-5
Jośko J, Gwóźdź B, Jedrzejowska-Szypułka H, Hendryk S (2000) Vascular endothelial growth factor (VEGF) and its effect on angiogenesis. Med Sci Monit 6:1047–1052
Hu Y, Tao R, Chen L et al (2021) Exosomes derived from pioglitazone-pretreated MSCs accelerate diabetic wound healing through enhancing angiogenesis. J Nanobiotechnol 19:150. https://doi.org/10.1186/s12951-021-00894-5
Galiano RD, Tepper OM, Pelo CR et al (2004) Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am J Pathol 164:1935–1947. https://doi.org/10.1016/S0002-9440(10)63754-6
Gonzalo-Gil E, Galindo-Izquierdo M (2014) Papel del factor de crecimiento transformador-beta (TGF-β) en la fisiopatología de la artritis reumatoide. Reumatol Clin 10:174–179. https://doi.org/10.1016/j.reuma.2014.01.009
Liarte S, Bernabé-García Á, Nicolás FJ (2020) Role of TGF-β in skin chronic wounds: a keratinocyte perspective. Cells 9:306
de Mayo T, Conget P, Becerra-Bayona S et al (2017) The role of bone marrow mesenchymal stromal cell derivatives in skin wound healing in diabetic mice. PLoS One 12:e0177533. https://doi.org/10.1371/journal.pone.0177533
Khayambashi P, Iyer J, Pillai S et al (2021) Hydrogel encapsulation of mesenchymal stem cells and their derived exosomes for tissue engineering. Int J Mol Sci. https://doi.org/10.3390/ijms22020684
Cases-Perera O, Blanco-Elices C, Chato-Astrain J et al (2022) Development of secretome-based strategies to improve cell culture protocols in tissue engineering. Sci Rep 12:10003. https://doi.org/10.1038/s41598-022-14115-y
McDonald TA, Zepeda ML, Tomlinson MJ et al (2010) Subcutaneous administration of biotherapeutics: current experience in animal models. Curr Opin Mol Ther 12:461–470
Zeng Q-L, Liu D-W (2021) Mesenchymal stem cell-derived exosomes: An emerging therapeutic strategy for normal and chronic wound healing. World J Clin Cases 9:6218–6233. https://doi.org/10.12998/wjcc.v9.i22.6218
Acknowledgements
The authors are grateful to Lorestan University for financial assistance.
Author information
Authors and Affiliations
Contributions
RI: Conducting experiments, statistic analysis, financing, writing and editing. SHH: Project administration, methodology, editing. SB: Methodology, editing, project consulting. We the undersigned declare that all authors have seen and approved the final version of the manuscript being submitted. We warrant that the article is the authors’ original work, has not published before and is not under consideration for publication elsewhere.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Izadi, R., Hejazi, S.H. & Bahramikia, S. Alternative viewpoint against diabetic wound based on stem cell secretome that can mediated angiogenesis and reduce inflammation. Arch Dermatol Res 316, 28 (2024). https://doi.org/10.1007/s00403-023-02739-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00403-023-02739-7